Novel immune checkpoint inhibitors
An immune checkpoint, inhibitory technology, applied in the field of prognostic biomarkers, can solve the problems of high treatment cost and toxicity, little patient benefit, no benefit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Lymphocyte activation gene 3 protein (LAG3) is a cell surface T-cell inhibitory protein and is thought to function by interacting with major histocompatibility complex class II (MHC-II). However, in various experimental settings, this interaction was not required for LAG3-induced inhibitory function, suggesting the existence of other functional LAG3 ligands. Using a genome-scale receptor array system, we identified fibrinogen-like protein 1 (FGL1), a liver-enriched soluble protein with mitogenic and metabolic functions in hepatocytes, as a LAG3-binding partner.
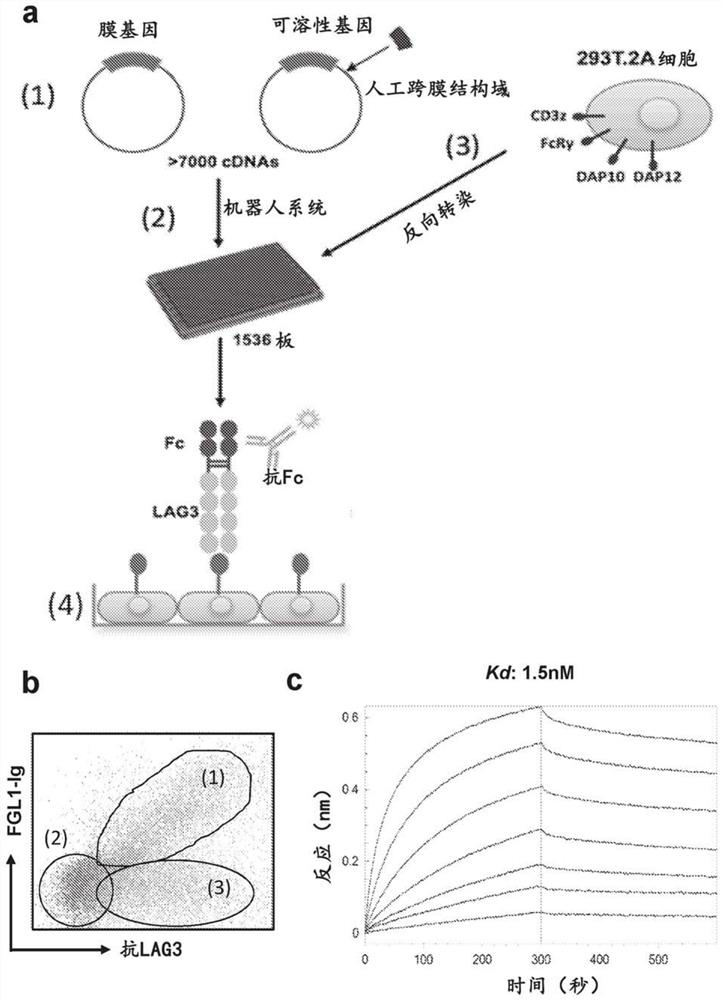
[0157] Using immunoglobulin (Ig) Fc-tagged LAG3 extracellular domain fusion proteins, the genome-scale receptor array platform (RAP) was used to search for novel LAG3-binding proteins ( figure 1 a). RAP is a semi-automated large-scale gene expression system in which individual human cDNAs encoding transmembrane and soluble proteins can be transiently overexpressed on the surface of 293T cells (Yao, S. et al. B...
Embodiment 2
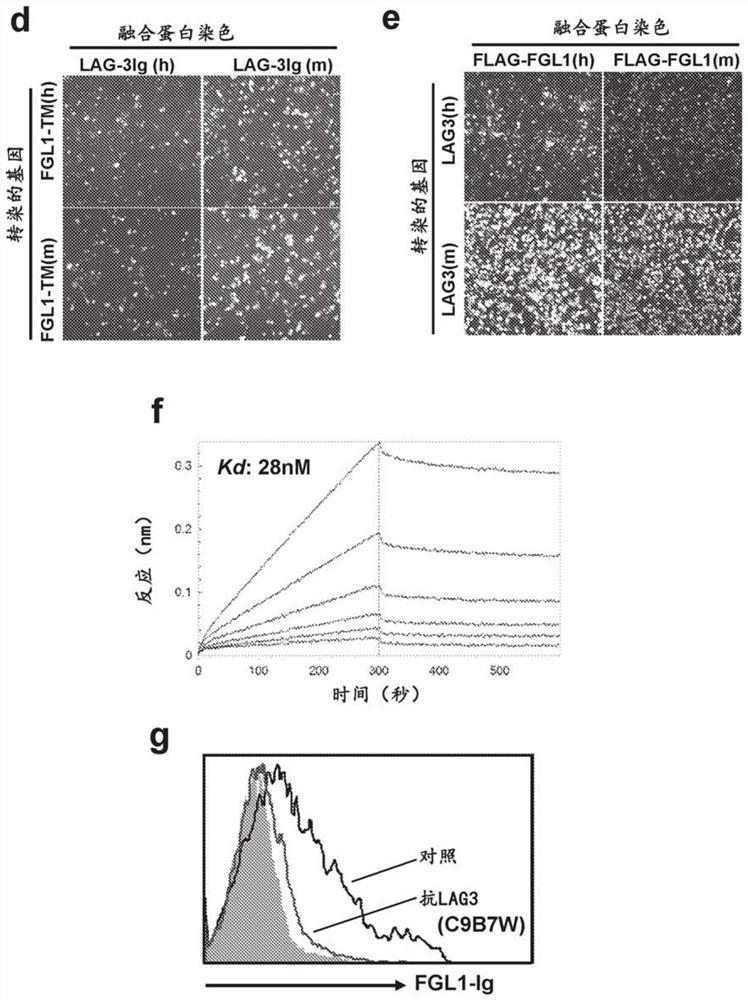
[0159] The interaction appears to be conserved between human and mouse species ( figure 1 d and figure 1 e). FGL1 / LAG3 interaction can be detected by flow cytometry ( figure 2 b) and Octet biolayer interferometry at about 1.5nM Kd ( figure 1 d) Validation performed to demonstrate high affinity interactions. The slow off-rates in both mouse and human binding suggest possible stability of this interaction ( figure 1 c and figure 1 f). Other FGL1 homologues involved in regulating Treg function, including FGL2, as well as several angiopoietin-related family members, were not shown to interact with LAG3 (data not shown), suggesting that the FGL1 / LAG3 interaction is highly specific.
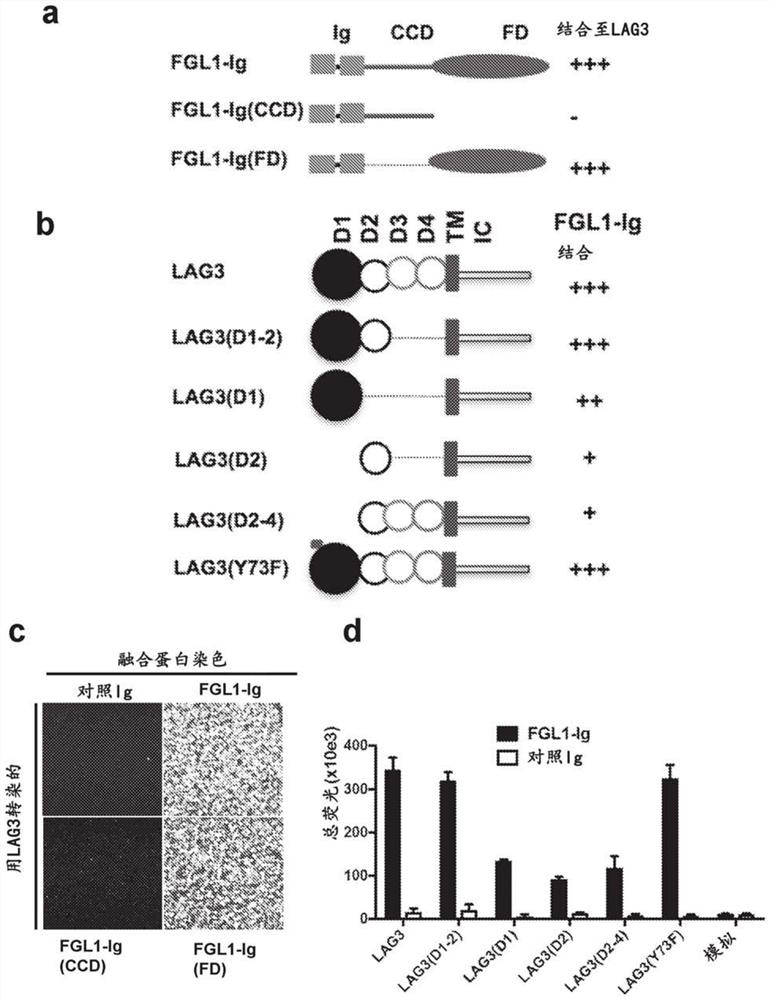
[0160] We worked to determine the binding domain of FGL1 to LAG3 and whether this affects MHC-II interaction. FGL1 consists of a helix-coil domain (CCD) and a fibrinogen-like domain (FD) (Yamamoto, T. et al. Molecular cloning and initial characterization of a novel fibrinogen-related gene, HFRE...
Embodiment 3
[0163] In a competition assay, it was further determined whether existing monoclonal anti-LAG3 antibodies blocked the FGL1-LAG3 interaction. Human LAG3-expressing CHO cells were treated with fluorescently labeled FGL1 (1 ug / ml FGL-A647) in the presence or absence of anti-LAG3 antibody (10 ug / ml anti-LAG3-A488 antibody; R&D Systems) or with anti-CD3 antibody Primary T cells activated to induce LAG3 expression were stained to determine whether they compete with FGL1 for binding to LAG3. Binding of FGL1 was not reduced in the presence of anti-LAG3 antibodies (data not shown) and thus did not appear to interfere with the FGL1:LAG3 interaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com