Methods of treating prostate cancer with an Anti- psma/cd3 antibody
A prostate cancer and antibody technology, applied in the direction of antibody medical components, anti-animal/human immunoglobulin, antibodies, etc., can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1. Materials
[0106] Generation of PSMA cell lines. Expression vectors displaying full-length chimpanzee PSMA (SEQ ID NO:2) or full-length cynomolgus monkey PSMA (SEQ ID NO:3) were generated for use as screening tools to evaluate anti-PSMA leads. The vector was transiently transfected into HEK293F cells. Transfected 293F suspension cells were plated in serum-supplemented growth medium, allowed to become adherent and selected for stable plasmid integration. Single-cell populations were selected by serial dilution, and (PSMAL Antibody (Center) affinity-purified rabbit polyclonal antibody (Cat. No. OAAB02483, Aviva Systems Biology) was used as the primary antibody, while R-PE anti-rabbit secondary antibody (Cat. -116-144, Jackson ImmunoResearch Laboratories, Inc.) and rabbit polyclonal IgG (Cat. No. SC-532, Santa Cruz Biotechnology) as an isotype control) were quantified by FACS for PSMA surface receptor expression.
[0107] SEQ ID NO:2 (full-length chimp...
Embodiment 2
[0114] Example 2. Generation of anti-chimpanzee and anti-human PSMA antibodies
[0115] Panning with recombinant protein. First solution panning of a de novo human Fab-pIX library consisting of VH1-69, 3-23, and 5-51 heavy chain libraries paired with libraries of four human VL germline genes (A27, B3, L6, O12) (Shi, L. et al., J Mol Biol, 2010.397(2): pp. 385-396, WO2009 / 085462) were performed using an alternate panning method in which strands coated with biotinylated chimpanzee PSMA ECD were prepared according to the manufacturer's protocol. One round of phage capture was performed on mycoavidin magnetic beads (Invitrogen, cat. no. 112.05D, lot no. 62992920), followed by a round of phage capture on cynomolgus PSMA-Fc-coated ProtG beads (Invitrogen, cat. no. 10003D) according to the manufacturer's protocol. Phage capture followed by phage capture on Sera-mag Double Speed Neutravidin magnetic beads coated with biotinylated chimpanzee PSMA ECD (Thermo, cat. no. 7815-2104-01...
Embodiment 3
[0134] Example 3. Production and Characterization of Anti-CD3 Antibodies
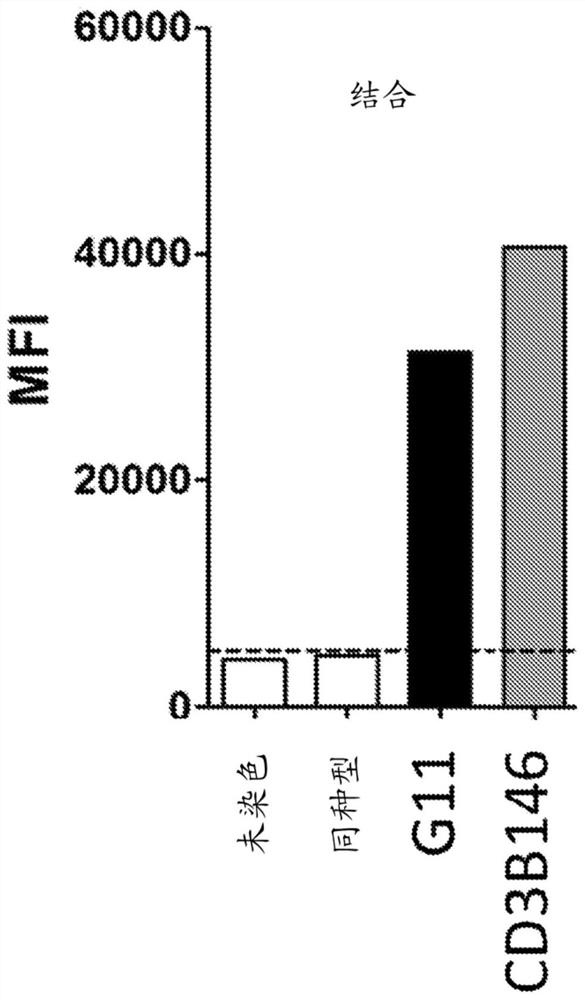
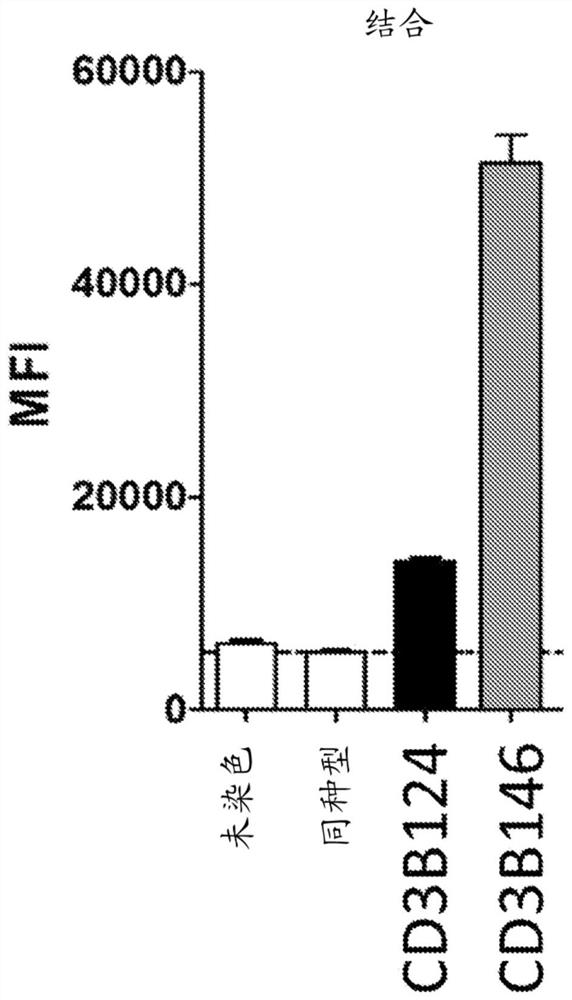
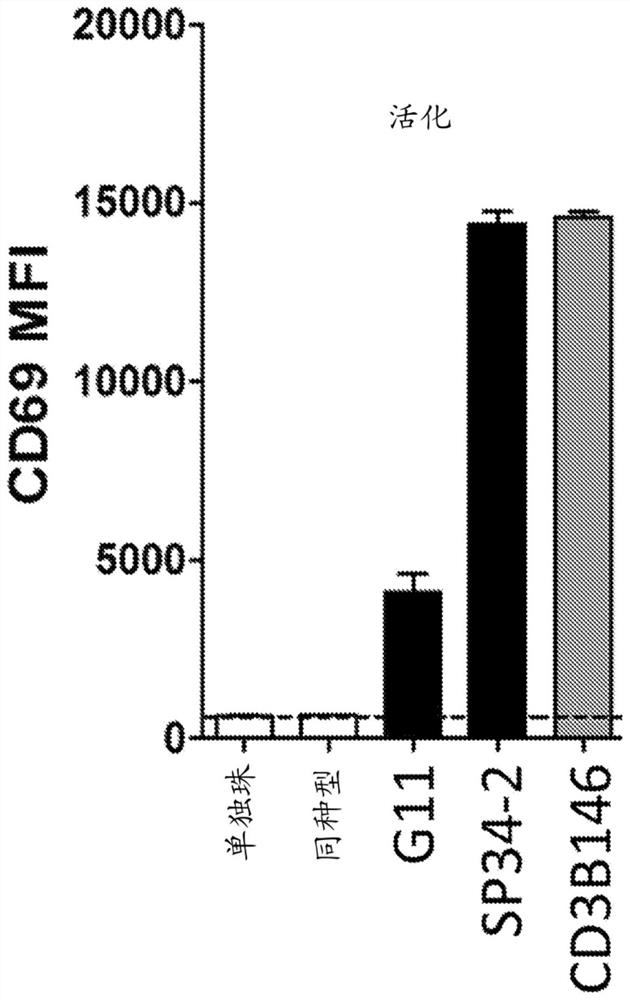
[0135] Production of anti-CD3 antibodies. The commercial anti-CD3 antibody SP34 (mouse IgG1 isotype anti-human CD3 IgG1 antibody) was humanized by the human framework adaptation method (Fransson et al., JMB, 2010398(2):214-31). To maintain the conformation of CDR-H3, the mouse residues at positions Val38, Gly48, Gly51 and V59 in VL and Ala at position 48 in VH were retained. Add these "back mutations" to your humanization plan. The resulting anti-CD3 variant was designated CD3B146.
[0136] Humanized anti-CD3 antibody binds endogenous cells of primary T cells. Binding of CD3B146 to cell surface CD3ε on primary human T cells and primary cynomolgus monkey CD4+ T cells was tested to assess retention of cross-reactivity. CD4+ T cells purified from peripheral blood of cynomolgus monkeys (Zen Bio, Triangle Research Park, USA) were used. Briefly, binding of anti-CD3 antibody to cell surface CD3ε was ass...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap