Pharmaceutical composition for preventing or treating obesity containing biglycan as active ingredient
A proteoglycan and active ingredient technology, which can be used in medical preparations containing active ingredients, drug combinations, peptide/protein ingredients, etc. clarify issues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Confirmation that biglycan suppresses the expression of appetite-stimulating peptide in vitro
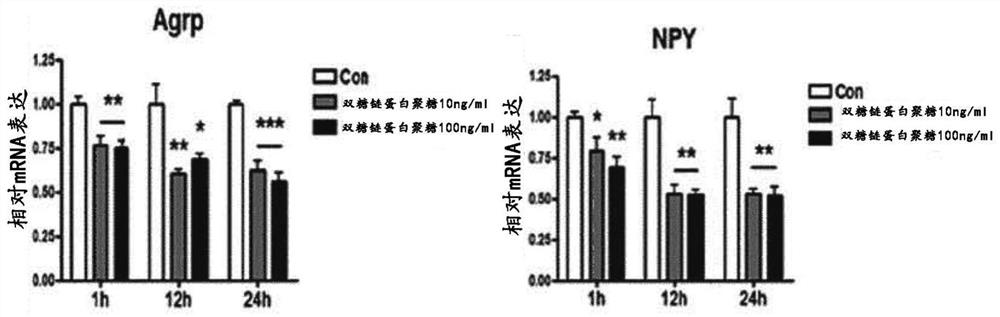
[0069] Hypothalamic cells N41 expressing appetite-stimulating peptides, agouti-related protein (AgRP) and neuropeptide Y (NPY) (Imperatore et al., Pharmacol. Res. 111:600-609, 2016; Oh et al., Autophagy 12:2009 -2025, 2016; Sasaki et al., Endocrinology 151:2556-2566, 2010, (Cellutions Biosystems Inc., CLU121)) in DMEM containing 10% FBS and 1% penicillin-streptomycin at 37°C and 5% CO 2cultivated in an atmosphere. Then, the cells were each treated with 0 ng / ml, 10 ng / ml and 100 ng / ml biglycan, and cultured for 1 hour, 12 hours and 24 hours. The control used here was the cells treated with the solution for dissolving biglycan (0.1% BSA). Then, mRNA expression was measured using real-time PCR.
[0070] Such as figure 1 As shown, the results showed that treatment with biglycan significantly decreased the mRNA expression of the appetite-stimulating peptides AgRP an...
Embodiment 2
[0071] Example 2: Confirmation that biglycan enhances the expression of appetite-suppressing peptides in vitro
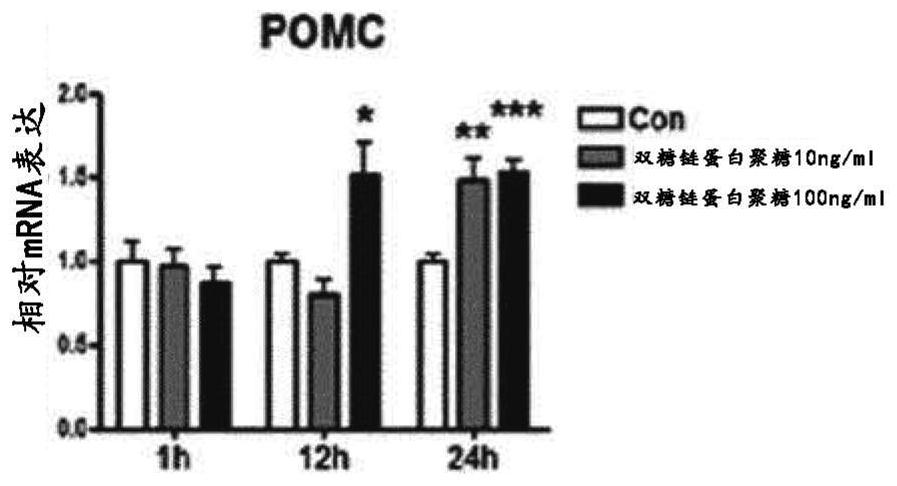
[0072] Hypothalamic cells expressing the appetite suppressant peptide proopiomelanocortin (POMC) N43 / 5 (Cai et al., J.Endocrinol.192:605-614, 2007; Cakir et al., The Journal of Biological Chemistry288:17675-17688 , 2013; Oh et al., Autophagy 12:2009-2025, 2016, (CellutionsBiosystems Inc, CLU121)) in DMEM containing 10% FBS and 1% penicillin-streptomycin at 37°C and 5% CO 2 cultivated in an atmosphere. Then, the cells were treated with 0 ng / ml, 10 ng / ml and 100 ng / ml biglycan, respectively, and cultured for 1 hour, 12 hours and 24 hours. The control used here was the cells treated with the solution for dissolving biglycan (0.1% BSA). Then, mRNA expression was measured using real-time PCR.
[0073] Such as figure 2 As shown, the results showed that treatment with biglycan significantly increased the mRNA expression of the appetite suppressant peptide POMC compare...
Embodiment 3
[0074] Example 3: Confirmation of the appetite-suppressing and weight-gain-suppressing effects of biglycan in an obesity-induced animal model
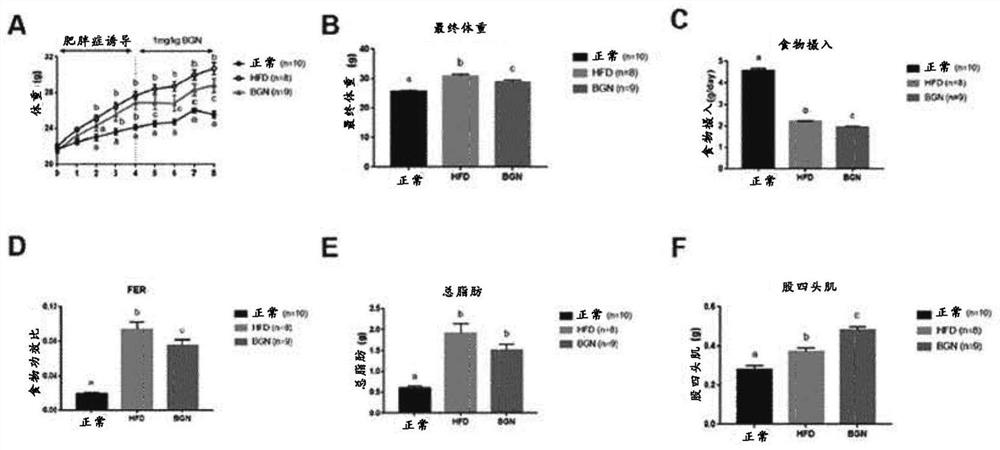
[0075] An obesity-induced animal model was generated and used to determine the role of biglycan in suppressing appetite and weight gain.
[0076] First, nine mice (C57BL / 6J males) were fed a high-fat diet (HFD) for 4 weeks to generate an obesity-induced animal model.
[0077] A biglycan-administered group was generated by intraperitoneally administering biglycan to each mouse once every two days at a dose of 1 mg / kg body weight.
[0078] Four weeks after induction of obesity, the difference between the high-fat diet (HFD) group and the high-fat diet group (BGN) administered with biglycan (biglycan was administered at a dose of 1 mg / Kg body weight) was measured. Difference in weight gain ( image 3 A). The final body weight of the biglycan-administered group (BGN) was significantly lower than that of the high-fat diet group (HFD) ( ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap