Application of Perospirone and derivative thereof in preparation of antitumor drugs
A technology of perospirone and its derivatives, which is applied in the field of biomedicine and can solve the problems of low induction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: CCK8 colorimetric method investigates the cytotoxicity of perospirone hydrochloride
[0065] Using CCK8 colorimetric method to compare the effect of perospirone hydrochloride on human breast cancer MDA-MB-231 and MDA-MB-231LM2 cells, ovarian cancer A2780 cells, lung cancer H1299 and A549 cells, colorectal cancer HCT116 and SW480 cells, liver cancer MHCC -97L and SMMC-7721 cells, gastric cancer SGC-7901 cells, melanoma A375 cells, pancreatic cancer Panc-1 cells, leukemia HL60 cells, lymphoma MINO cells, esophageal cancer EC109 cells, thyroid cancer TPC-1 cells, nasopharyngeal carcinoma Effect of cytotoxic perospirone hydrochloride on growth inhibition of 5-8F cells, renal clear cell adenocarcinoma 786-O cells, glioma U251 cells, cervical cancer Siha cells, and prostate cancer PC-3 cells. Various cells were seeded in a 96-well plate at a density of 8000 cells / well. After 24 hours of seeding, the drugs were diluted to 0, 0.01, 0.1, 1, 10, 20, 40, 80, 160, 320...
Embodiment 2
[0069] Example 2: In vitro tumor growth inhibition experiment
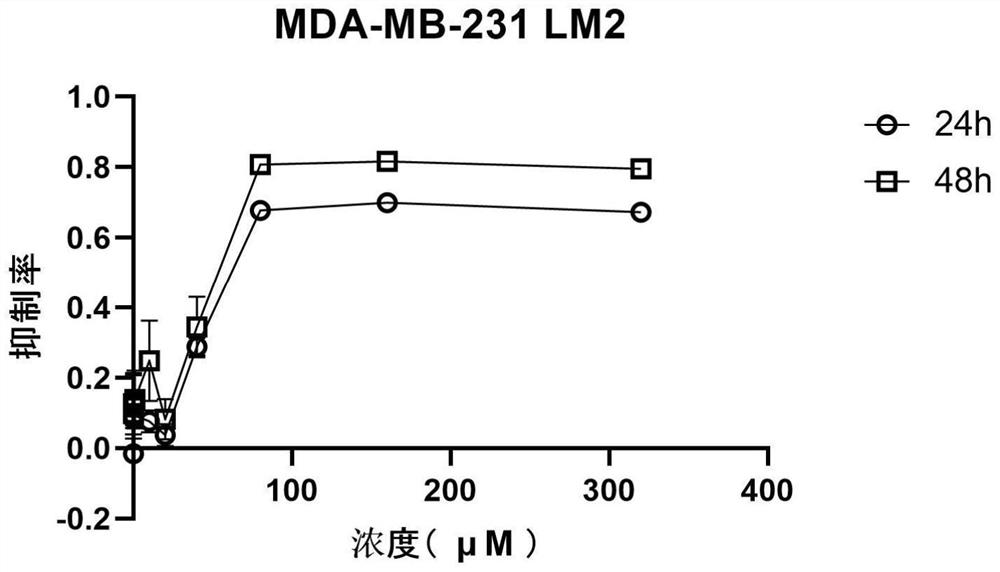
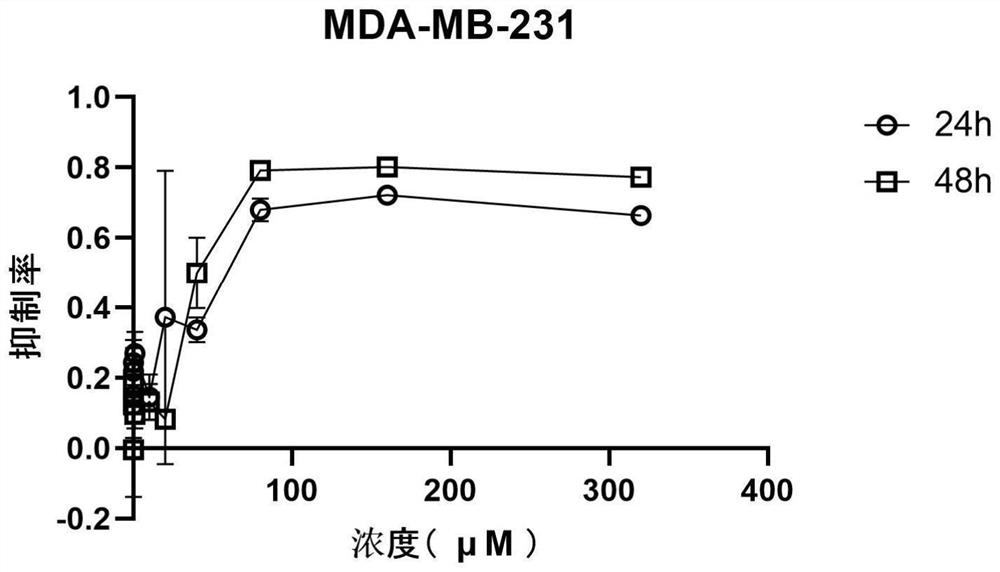
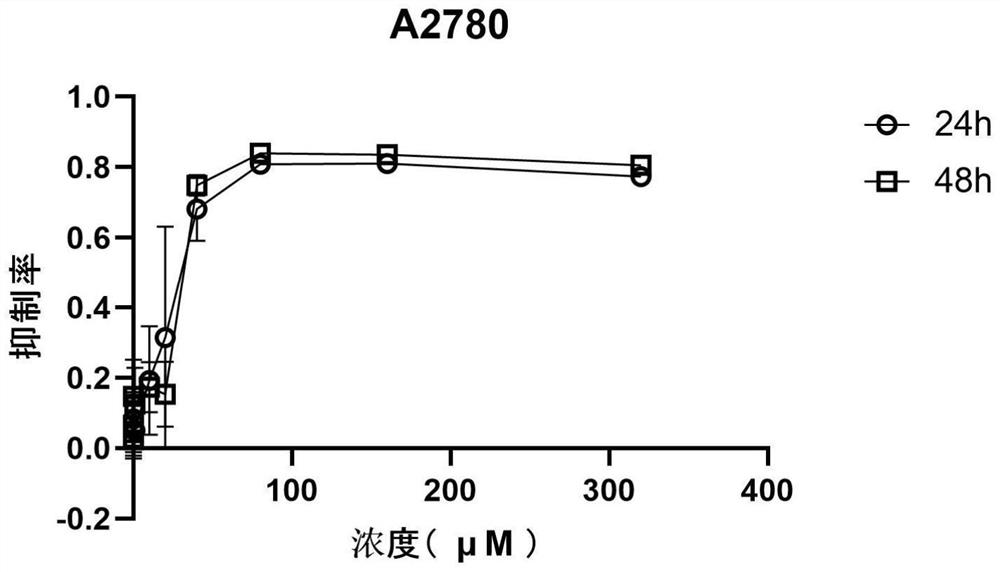
[0070] see results Figure 1-21 , after 320μM perospirone hydrochloride acted on different tumor cells for 48h, the inhibition rates to tumor cells were 79.52±1.17% (breast cancer cell MDA-MB-231LM2, such as figure 1 shown), 77.24±0.24% (breast cancer cell MDA-MB-231, such as figure 2 shown), 89.35±0.83% (human colorectal cancer cell HCT116, such as Figure 6 shown), 83.40±0.28% (colon cancer SW480 cells, such as Figure 7 shown), 80.56±1.08% (human ovarian cancer A2780 cells, such as image 3 shown), 90.08±0.20% (lung adenocarcinoma cell H1299, such as Figure 5 shown), 89.42±0.54% (lung adenocarcinoma cell A549, such as Figure 4 shown), 92.12±0.25% (hepatoma cell MHCC-97L, such as Figure 8 shown), 89.82±1.07% (liver cancer cell SMMC-7721, as Figure 9 shown), 89.99±0.61% (gastric adenocarcinoma SGC-7901, such as Figure 10 shown), 74.65±0.56% (melanoma A375, such as Figure 11 shown), 83.07±1.36% (panc...
Embodiment 3
[0071] Example 3: Effect of Perospirone Hydrochloride on Tumor Growth in Nude Mice Bearing Human Breast Cancer Cell MDA-MB-231LM2 Orthotopic Transplantation
[0072] Adapt 6-8 week-old BALB / C female nude mice to the environment for about 1 week, digest MDA-MB-231 LM2 cells and A549 cells with trypsin, centrifuge at 1000rpm for 5min, add 5mL of fresh DMEM without FBS, wash once and count , adjust the concentration to 2×10 7 cells / mL, inoculated into fat pads of mice in 100 μl each. Wait for the tumor volume to grow to 1-20mm 3 After starting the drug. Each type of cell was randomly divided into 2 groups, 10 in each group, which were the perospirone hydrochloride (5 mg / kg) administration group and the solvent blank control group. Nude mice were numbered, and after the tumor was inoculated, intraperitoneal injection of perospirone hydrochloride was carried out by group, once a day, and each mouse was given 100 μL of medicine (1 mg / ml dissolved in 5% DMSO+30% PEG300+ 10% Tween...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap