Methods and compositions for improving enamel hardness and resistance
A technology of composition and mixture, which is applied in the direction of pharmaceutical formulations, cosmetic preparations, cosmetic preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0130] Generalized Solution Process
[0131] All glassware was cleaned with a 1% Alconox solution, rinsed three times in tap water, three times in 1M-ohm residential DI water, and finally three times in 18.2M-ohm Millipore water. The glassware was air dried overnight at 20°C. Place 450 mL of 18.2M-ohm Millipore water in a beaker with a stir bar. Add calcium source and phosphate source to beaker with water, start stir bar. For example, in Example 1, calcium hydrogen phosphate anhydrous (CaHPO 4 ) with a target concentration of 0.01M. CaHPO 4 Acts as a source of calcium and phosphate. Therefore, in Example 1, the CaHPO of 0.6803g 4 Add to beaker. In all examples, due to undissolved and suspended CaHPO 4 , the solution is cloudy.
[0132] A pH meter (719S Titrino, Metrohm AG, Herisau, Switzerland) was calibrated by testing two solutions with a known pH between pH 3 and pH 7 according to the manufacturer's instructions. Adjust the suspended CaHPO by slow dropwise addit...
Embodiment 1
[0240]
Embodiment 2
[0242]
[0243] Table 3. Precipitated Coatings
[0244]
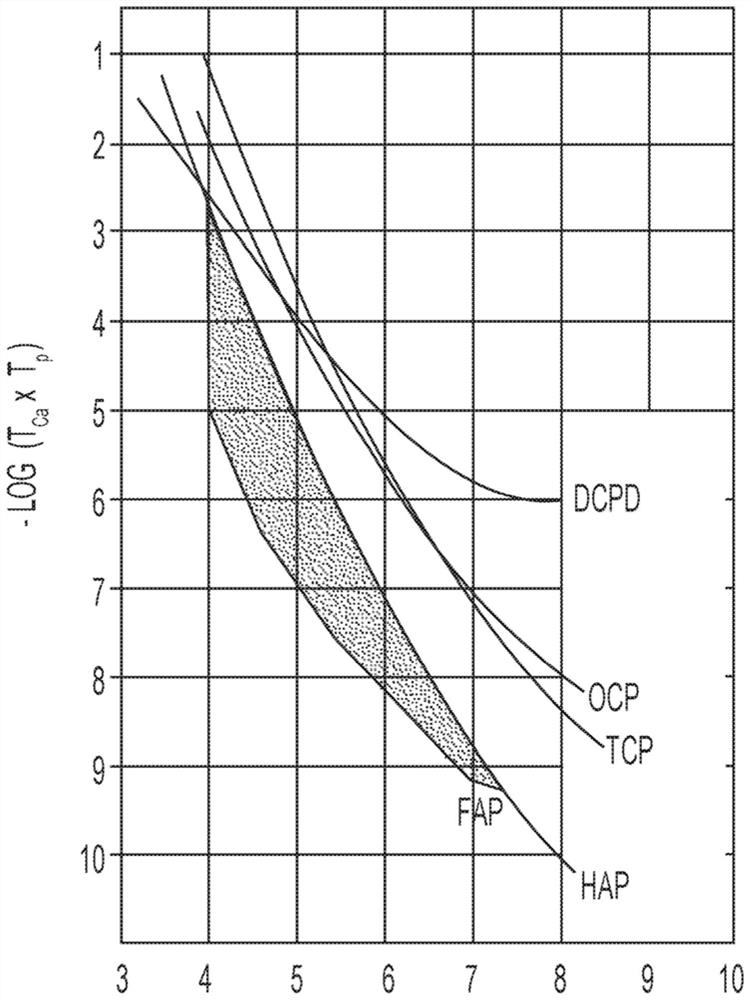
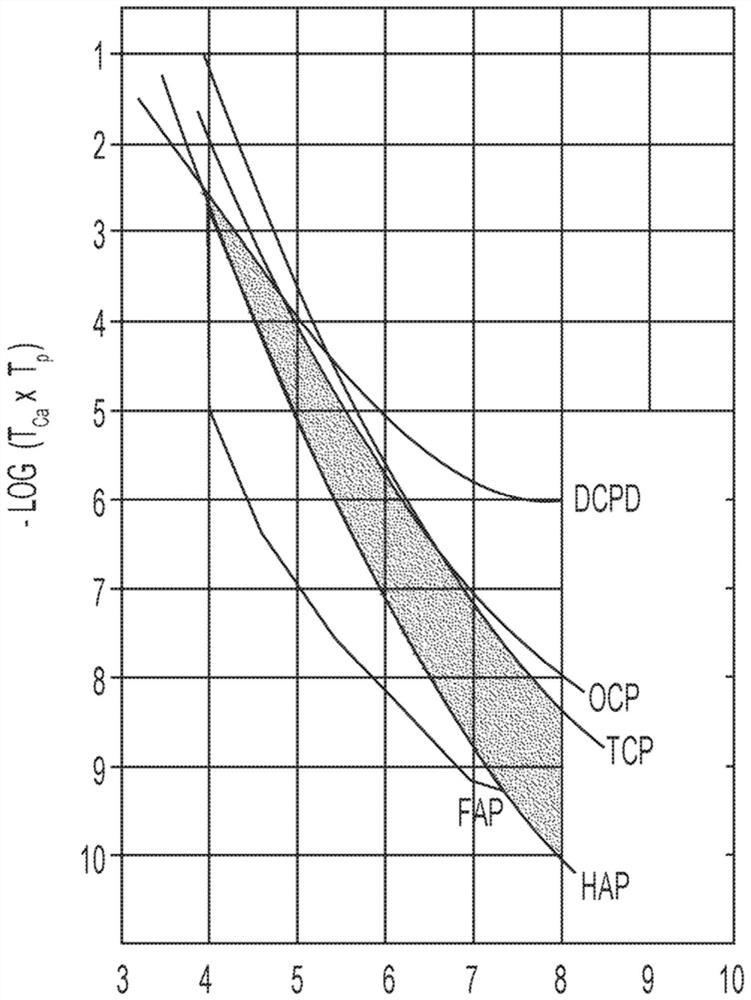
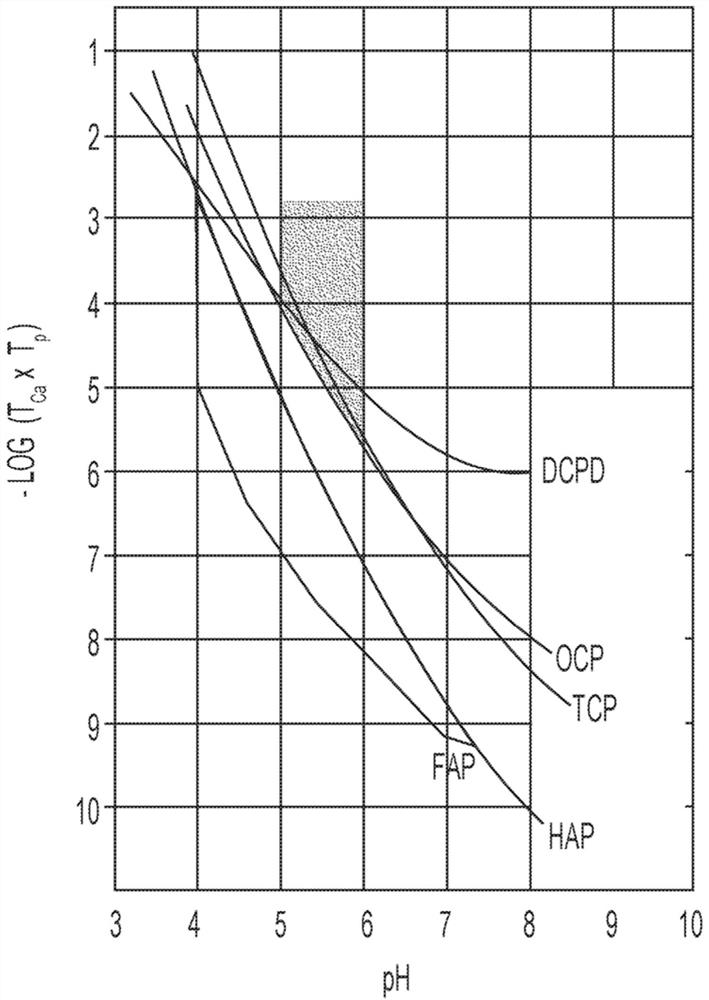
[0245] Table 3 shows the concentrations of calcium and phosphate ions that can form precipitates on the tooth enamel surface (expressed as -log([Ca 2+ ]×[PO 4 3- ])value. For example, no precipitate was formed in Comparative Examples 1-17, despite showing increased resistance to corrosive acids and / or carious acids. Comparative Examples 18-20 precipitated calcium phosphate compounds on the tooth enamel surface, but within about 16 hours (and / or overnight). Surprisingly, corresponding to image 3 Invention Example 1 and Invention Example 2 of the shaded area precipitate calcium phosphate compounds on the tooth enamel surface in 1 hour or less. Has the same pH as Inventive Examples 1 and 2, but has a slightly lower -log([Ca 2+ ]×[PO 4 3- ]) value of Comparative Example 21 did not form a precipitate coating on the tooth enamel surface. Thus, although a -log([Ca 2+ ]×[PO 4 3- ]) values did not produce ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com