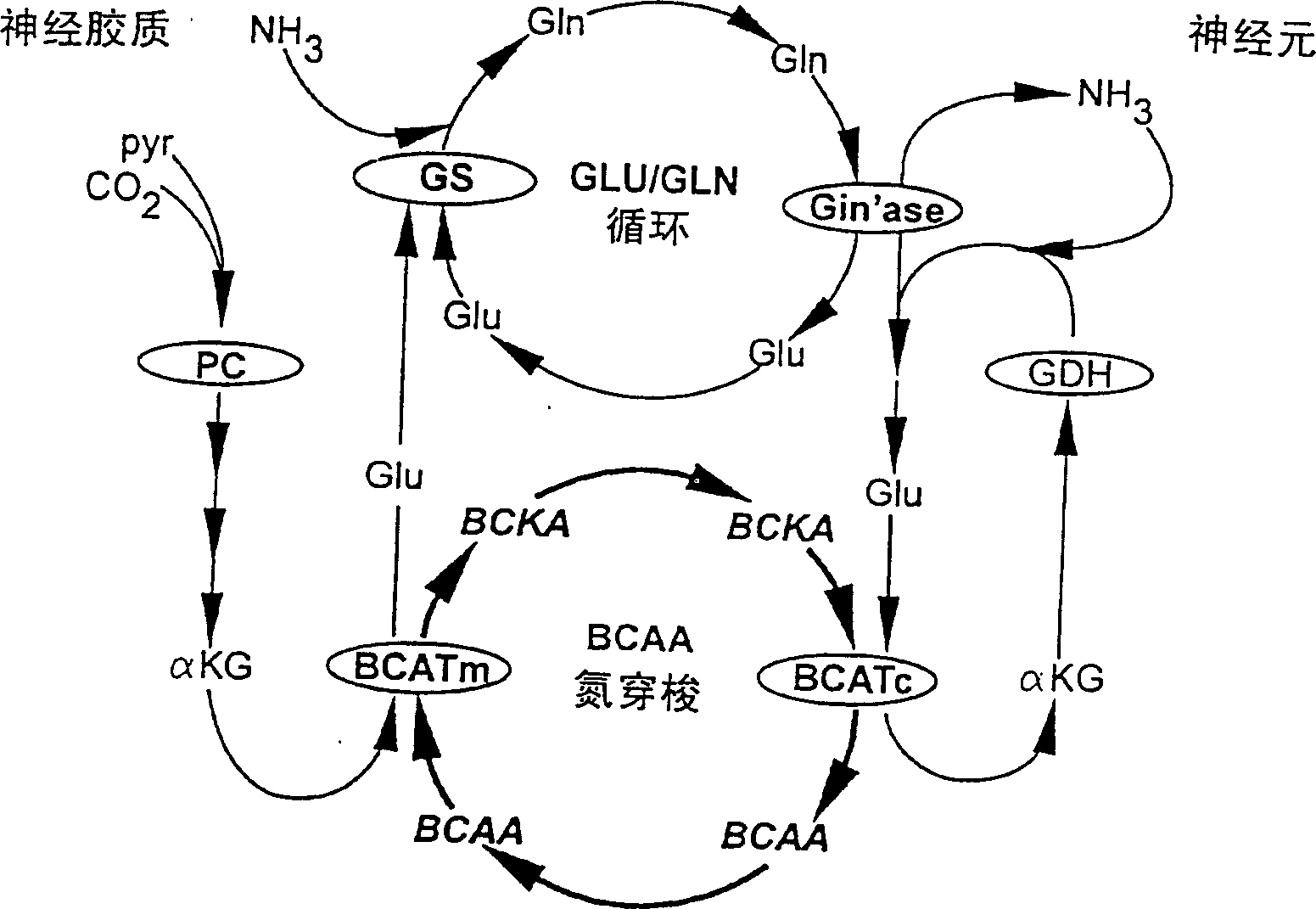
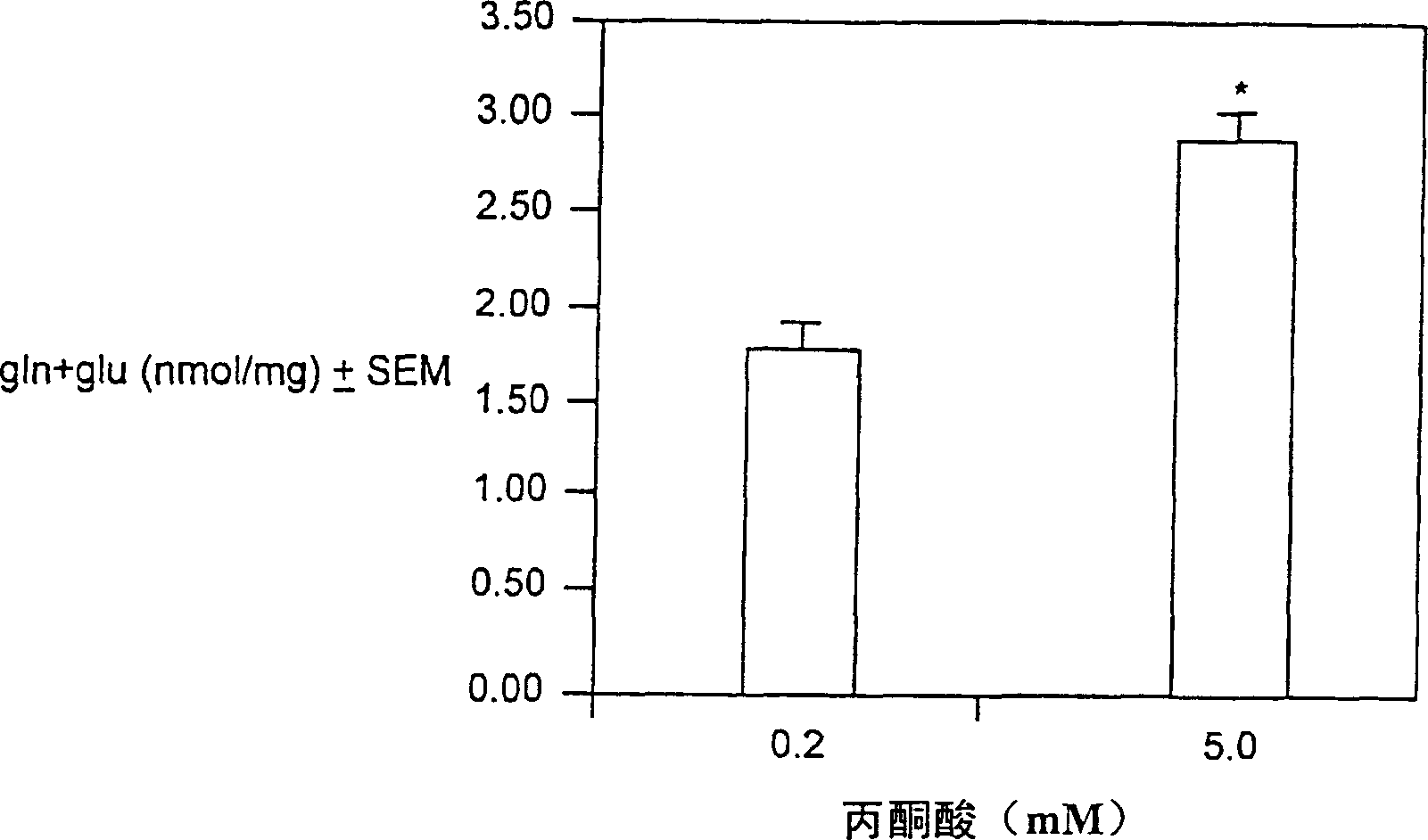
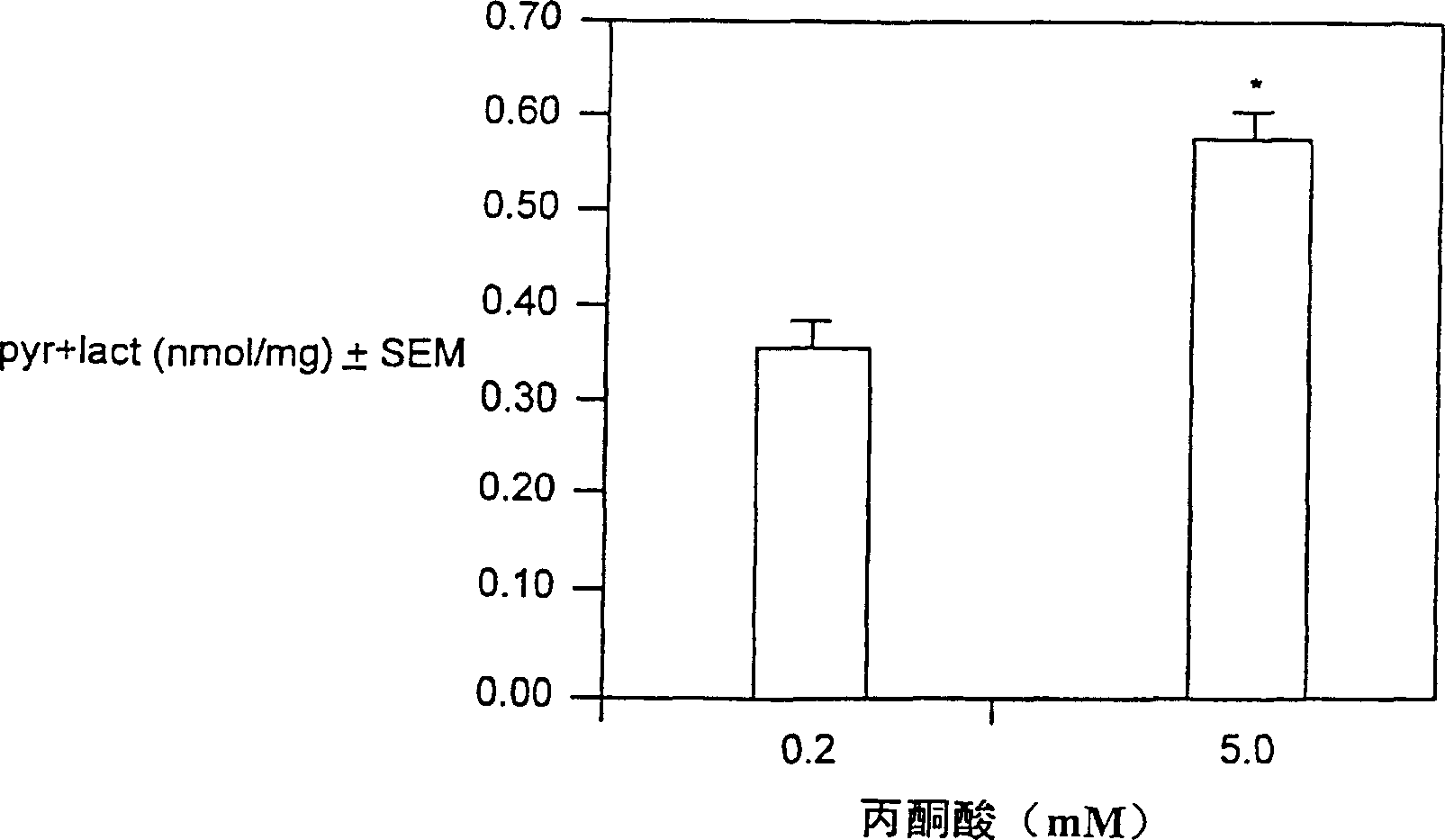
Branched chain amino acid-dependent aminotransferase inhibitors and their use in treatment of diabetic retinopathy
A technology of alkylcycloalkyl and dimethylcyclohexyl, which is applied in the field of prevention and treatment of diabetic retinopathy, and can solve problems such as inactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Example 1: trans-(1R,3R)(1-aminomethyl-3-methylcyclohexyl)acetic acid hydrochloride
[0269]
[0270]
[0271] (i) EtO 2 CCH 2 CN, NH 4 Ac, AcOH, toluene, 120°C
[0272] (ii) a. NaCN, EtOH (95%), H 2 O, 115°C, b.HCl (g)
[0273] (iii) EtOH, HCl (g), toluene
[0274] (iv) HCl, H 2 o
[0275] (v)H 2 , EtOH / NH 3 , Raney Ni, 30-50°C
[0276] (vi) HCl, H 2 O, 140°C
[0277] Step i: Ethyl 2-cyano-((R)-3-methylcyclohexylene)acetate
[0278] A mixture of 3-(R)-methylcyclohexanone (125mmol), ethyl cyanoacetate (124mmol), ammonium acetate (12.5mmol) and glacial acetic acid (24mmol) was refluxed in a Dean-Stark trap 24 hours. The mixture was cooled and washed with water. The aqueous washes were extracted with toluene. The toluene extract was combined with the original organic layer, dried over magnesium sulfate, and the solvent was evaporated. The oily crude product was purified by Kugelrohr d...
Embodiment 2
[0322] Example 2: (1-aminomethyl-2-methylcyclohexyl) acetic acid hydrochloride
[0323]
[0324] (i) EtO 2 CCH 2 CN, NH 4 Ac, AcOH, toluene, 120°C
[0325] (ii) a. NaCN, EtOH (95%), H 2 O, 115°C, b, HCl (g)
[0326] (iii) EtOH, HCl (g), toluene
[0327] (iv) HCl, H 2 o
[0328] (v) H 2 , EtOH / NH 3 , Raney Ni, 30-50°C
[0329] (vi) HCl, H 2 O, 140°C
[0330] Step i: Ethyl 2-cyano-(2-methylcyclohexylene)acetate
[0331] Following the general procedure (Example 1), step i, the reaction was carried out using (+ / -) 2-methylcyclohexanone (80 mmol), ethyl cyanoacetate (80 mmol), ammonium acetate (8 mmol) and glacial acetic acid (16 mmol) , a clear oil was obtained. Yield: 76%.
[0332] 1 HNMR (CDCl 3 )400MHzδ: 1.23 (3H, dd, J = 7, 10Hz), 1.35 (3H, t, J = 7Hz), 1.55-1.82 (5H, m), 1.93-2.05 (1H, m), 2.17 (1H, dt , J=5, 14Hz), 2.47 (1H, dt, J=5, 9Hz), 2.92-2.97 (1H, Brd, J=15Hz), 3.30-3.35 (1H, m), 3.81-3.86 (1H, Br d, J=15...
Embodiment 3
[0376] Example 3 (1-aminomethyl-3,3-dimethylcyclohexyl) acetic acid hydrochloride
[0377]
[0378]
[0379] i) CuI, MeLi, NH 4 Cl, NH 3 , (92%)
[0380] ii) NCCH 2 CO 2 Et, NH 4 OAc, AcOH, Toluene, (83%)
[0381] iii) NaCN, EtOH, H 2 O, (57%)
[0382] iv) HCl, EtOH, toluene, (93%)
[0383] v) H 2 , Raney Ni, EtOH, NH 3 , (84%)
[0384] vi) HCl, H 2 O, (64%)
[0385] Step i: 3,3-Dimethylcyclohexanone
[0386] The synthesis was carried out by the method described by Pelletier, S.W., Mody, N.V., J. Org. Chem., 1976.41, 1069.
[0387] A lithium dimethylcuprate solution was prepared by adding methyllithium (1.4M in diethyl ether, 77.25 mL, 2.45 mol) to copper(I) iodide (8.8 g, 0.046 mol) under argon atmosphere. The solution was cooled to 0° C., and 3-methylcyclohexen-1-one (5 mL, 0.044 mol) was added dropwise with stirring to form a dark yellow precipitate. The suspension was stirred at r...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap