Use of isoquinoline derivatives for treating cancer and MAP kinase related diseases
A technology for kinases and uses, which is applied in the field of isoquinoline derivatives for the treatment of cancer and MAP kinase-related diseases, and can solve the problems of no isoquinoline compounds and no therapeutic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
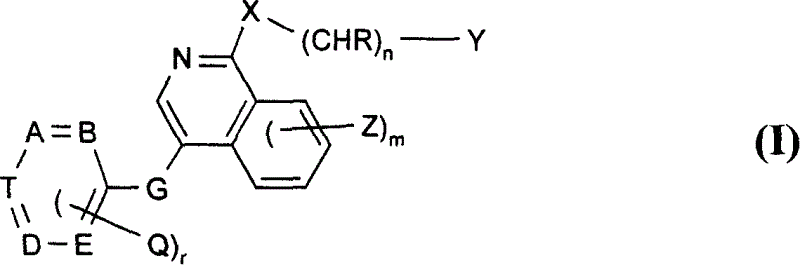
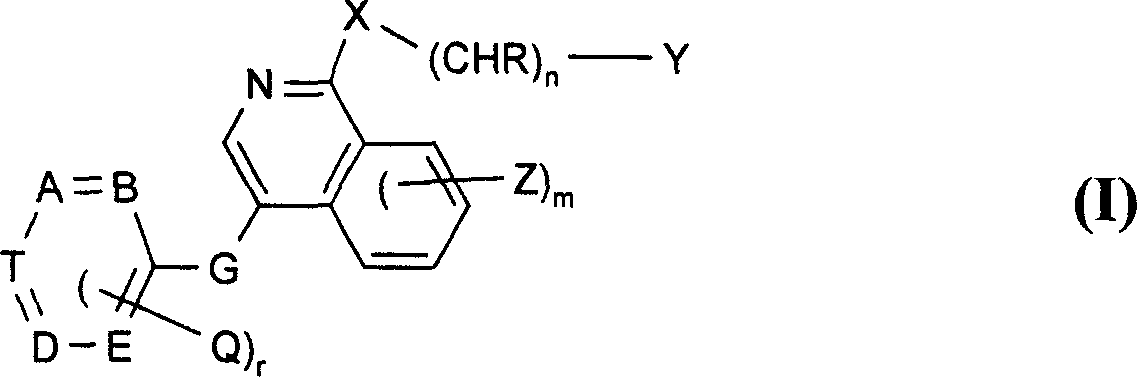
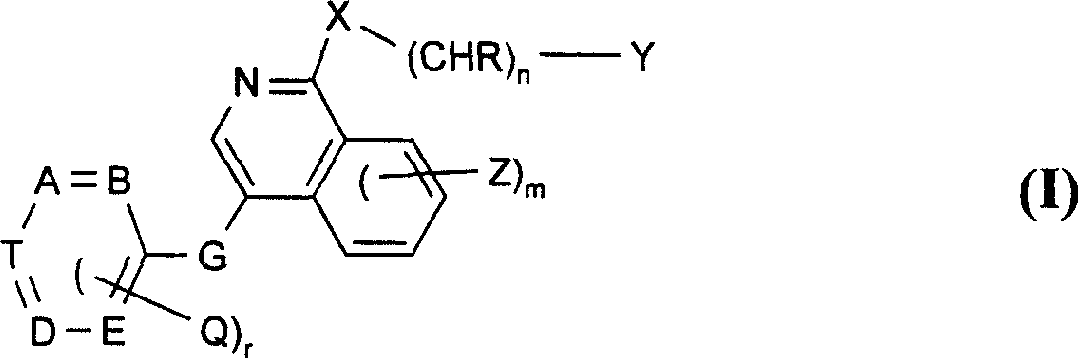
[0196]In the preparation of raw materials, if necessary, any functional groups that should not participate in the reaction are in a protected form. The preferred protecting groups, their introduction and their removal are described in method a) or the examples. Salts of the raw materials and intermediates may be used instead of the raw materials and intermediates to perform the reaction, provided that a group forming a salt exists, and the reaction may also be performed using a salt. Therefore, any raw materials mentioned above and below are also intended to include their salts, as long as they are advantageous and possible.
[0197] For G is -CH 2 -O-, -CH 2 -S-, -CH 2 -NH-, oxa, thia or -NR- and other symbols are as defined in formula I for the preparation of compounds of formula II, for example, by reacting a compound of formula IV with a compound of formula V, preferably with a compound of formula II Under the conditions similar to those mentioned in the reaction method a) of ...
Embodiment S1
[0226] Example S1: 1-(3,5-dimethylanilino)-4-[(pyridin-4-yl)-methyl]-isoquinoline (=N-(3,5-dimethylphenyl) )-[4-(pyridin-4-ylmethyl)-isoquinolin-1-yl]-amine)
[0227] Under anhydrous conditions, 100 mg (0.825 mmol) of 3,5-dimethylaniline was dissolved in 4 ml of ethanol, and 196 μl (0.784 mmol) of HCl (present in dioxane at a concentration of 4N) was added. After adding 200 mg (0.785 mmol) of 1-chloro-4-[(pyridin-4-yl)-methyl]-isoquinoline, the mixture was heated at 90°C for 8 hours. Then it is concentrated by evaporation; the residue is placed in 4ml water, 1ml saturated ammonia solution and 20ml CH 2 Cl 2 And separate the organic phase with Na 2 SO 4 (Anhydrous) Dry and concentrate again by evaporation. After column chromatography (SiO 2 ; Ethyl acetate / hexane 3:1) and crystallization from ethyl acetate / hexane to obtain the title compound: m.p.156-158°C; FAB-MS: (M+H) + =340; Anal.calc.(C 23 H 21 N 3 ·0.1H 2 O) C 80.95%, H 6.26%, N 12.31%; found C 80.9%, H 6.2%, N 12.4%.
[0228...
Embodiment S2
[0239] Example S2: 1-(3-Chlorobenzylamino)-4-[(pyridin-4-yl)-methyl]-isoquinoline
[0240] Under anhydrous conditions, 1.6ml (13.1mmol) of 3-chlorobenzylamino and 800mg (3.14mmol) of 1-chloro-4-(pyridin-4-ylmethyl)-isoquinoline (Example 1e) in 150 Stir at °C for 2 hours. The mixture was suspended in ethyl acetate, 1 ml of concentrated ammonia solution was added, washed with water and brine and the organic phase was dried (Na 2 SO 4 ) And concentrated by evaporation. Column chromatography (SiO 2 ; Ethyl acetate) to obtain the title compound: m.p.141-142°C; FAB-MS: (M+H) + =360; Anal.calc.(C 22 H 18 N 3 Cl) C 73.43%, H 5.04%, N 11.68%, Cl 9.85%; found C 73.2%, H 5.1%, N 11.6%, Cl 9.9%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap