Active segment of HER2/neu with Herstatin interaction, coding gene and application thereof
An active fragment, coding gene technology, applied in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of inhibition of cell proliferation, reduced ability of clone formation, unclear Herstatin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
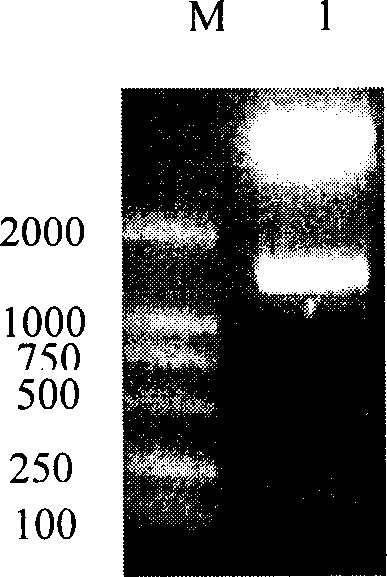
[0052] Embodiment 1, HER2 / neu interacts with Herstatin active fragment
[0053] 1. Cloning and expression of Herstatin gene
[0054] Transfection reagent Lipofectamine TM and pcDNA3.1 / Myc-His (-) are Invitrogen products; Restriction enzymes are purchased from New England Biolabs; T 4 DNA ligase was purchased from Promega; Pyrobest DNA polymerase and DNA molecular weight standard DL2000 were TaKaRa products; DNA fragment recovery kit and plasmid recovery kit were purchased from Biotech; primers were synthesized by Borun Biological Co., Ltd.
[0055] 1. Construction of pcDNA3.1(-)myc-his / Hst vector
[0056] According to the method introduced by Doherty et al. (Doherty JK, Bond C, Jardim A, Adelman JP, Clinton GM.1996.The ER-2 / neu receptor tyrosine kinase gene encodes a secreted autoinhibitor.Proc Natl Acad Sci USA 96:10869-10874) The full-length cDNA of Herstatin (clone number gi|10181232|gb|AF177761.2|AF177761 ) was cloned between the restriction endonuclease XhoI and Hin...
Embodiment 2
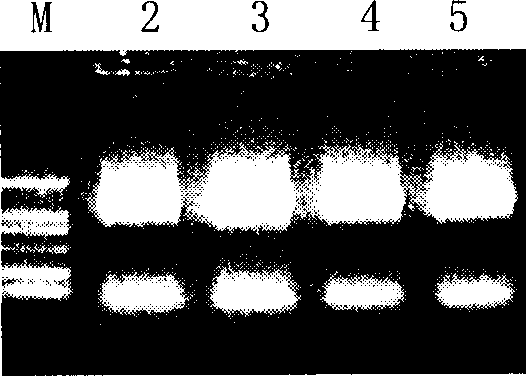
[0092] Example 2. Co-immunoprecipitation experiments verify the active fragments of interaction between HER2 / neu and Herstatin
[0093] In order to further verify the correctness of computer modeling. This model was further verified by co-immunoprecipitation of Herstatin and HER2 / neu mutants.
[0094] Material
[0095] Transfection reagent Lipofectamine TM For Invitrogen products; pcDNA3.1(-)myc-his / Hst, vectors pEGFP / ECDt, pEGFP / mE1 and pEGFP / mE2 according to literature (Wang JN, Feng JN, YuM, Xu M, Shi M, Zhou T, Yu XD , Shen BF, Guo N.2004. Structural analysis of the epitopes on erbB2 interacted with inhibitory or non-inhibitory monoclonal antibodies. Mol Immunol 40: 963-969) method construction. proteinA / G plusagarose beads, Santa Cruz products. Cocktail, Roche product. anti-Herstatin, mouse monoclonal antibody CW001, upstate product; GFP rabbit polyclonal antibody, product of BioVision.
[0096] will be 3×10 6 CHO cells were plated on a 10 cm culture plate and tran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com