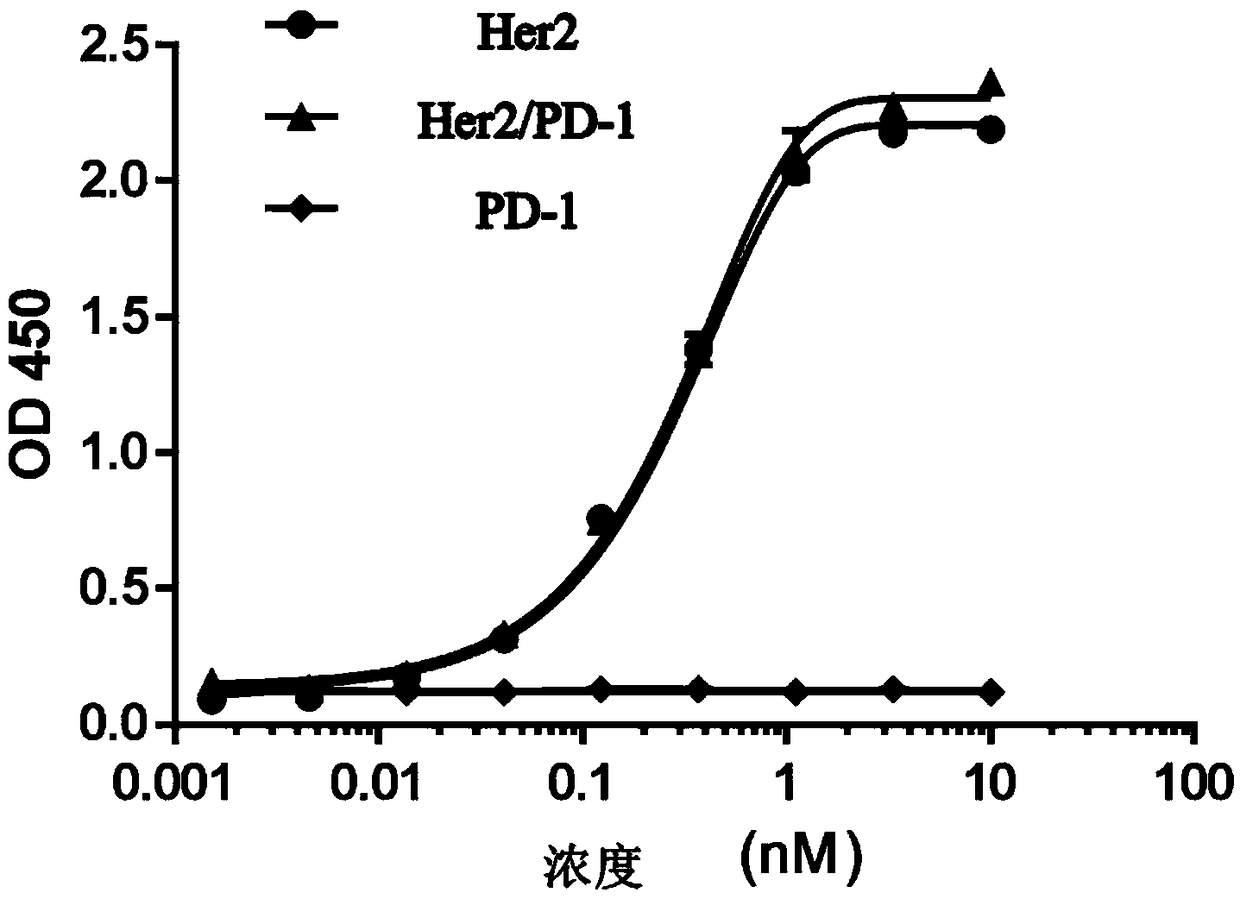
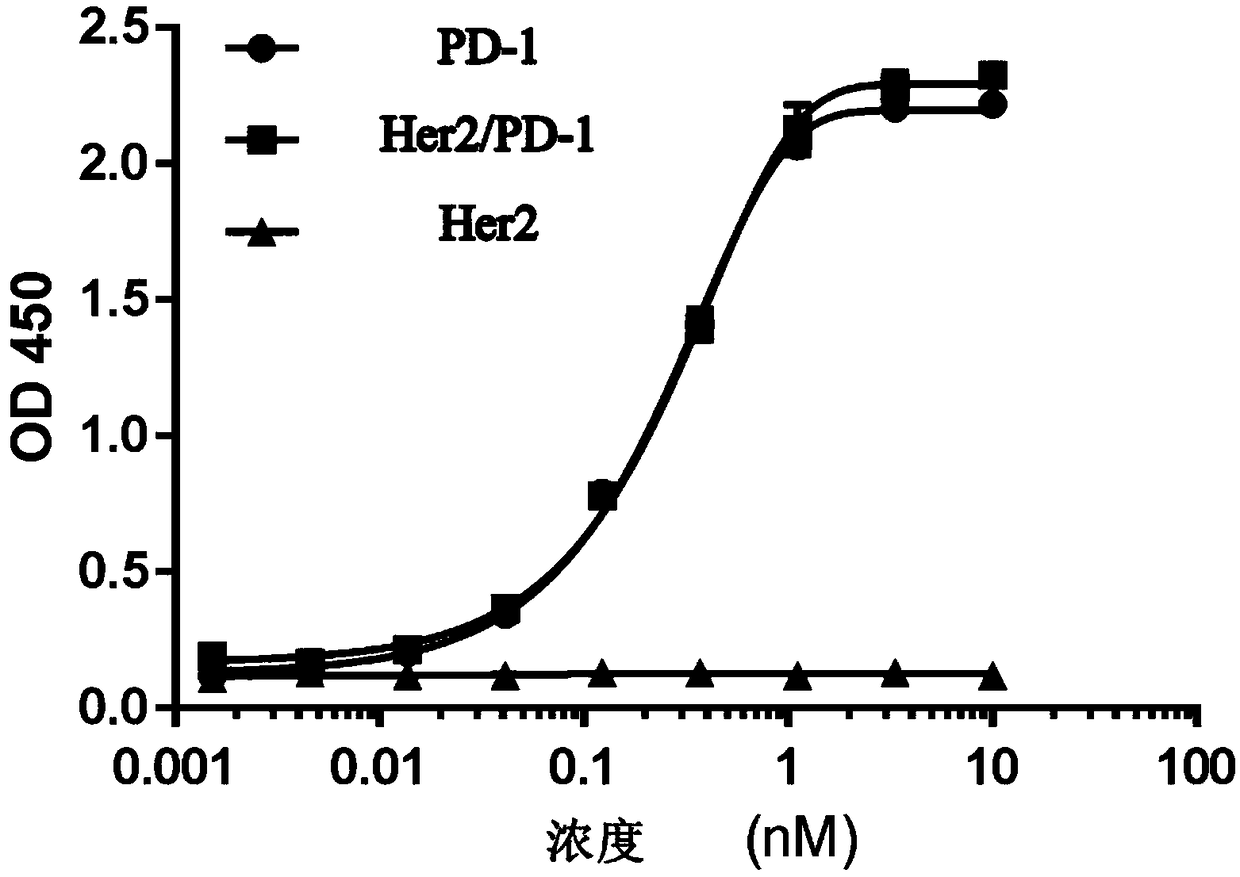
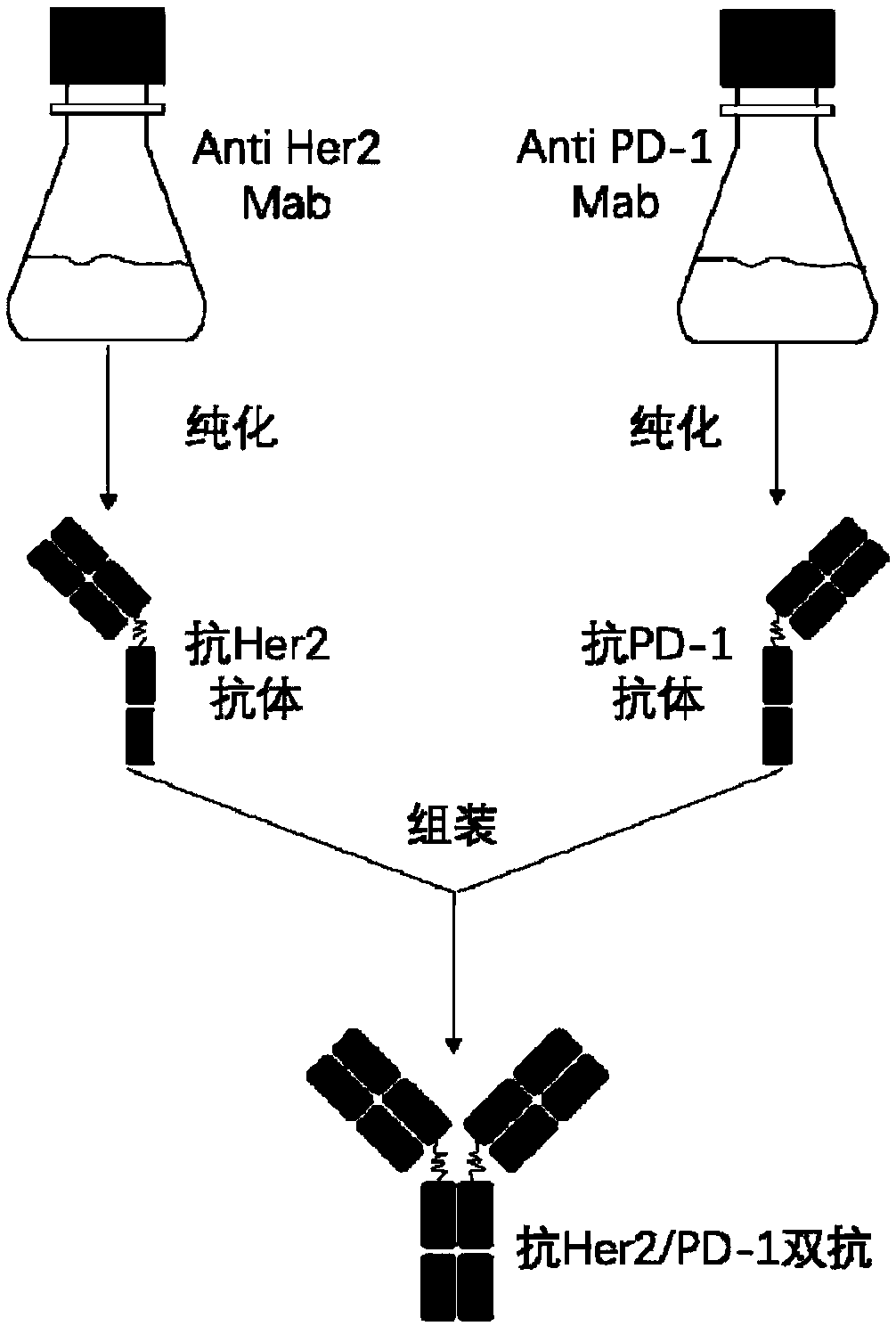
Anti-Her2/PD-1 bispecific antibody and preparation method thereof
A bispecific antibody, PD-1 technology, applied in the direction of antibodies, chemical instruments and methods, specific peptides, etc., to achieve the effect of reducing the formation of homodimers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Construction of Anti-Her2 Antibody and Anti-PD-1 Antibody Expression Vectors
[0030]The sequence of the full-length gene (light chain and heavy chain) of the fully human anti-Her2 monoclonal antibody adopts the sequence published by Trastuzumab (IMGT INN 7637_H, INN7637_L), see SEQ ID NO.1 for the heavy chain, and SEQ ID NO.2 for the light chain . The Fab (light chain and heavy chain) sequence of the fully human anti-PD-1 monoclonal antibody adopts the sequence published by Pembrolizumab (IMGT INN 9798_H, INN9798_L), see SEQ ID NO.3 for the heavy chain, and SEQ ID NO.4 for the light chain , Fc adopts the Fc sequence of Trastuzumab. Carry out charge analysis on the Fc amino acid ammonium sequences of Trastuzumab and Pembrolizumab, and carry out amino acid modification on the CH3 region. The modified Trastuzumab heavy chain amino acid sequence is SEQ ID NO.5, and the modified Pembrolizumab heavy chain amino acid sequence is SEQ ID NO.6 . Amino acid modificat...
Embodiment 2
[0034] Example 2. Construction of anti-HER2 antibody and anti-PD-1 antibody cell lines
[0035] The anti-PD-1 antibody and anti-Her2 antibody expression plasmids were transferred into CHO-S host cells by electroporation kit (AmaxaTM cell line NucleofectorTM Kit V) and single-well cell electroporation instrument (NucleofectorTM 2b, Lonza), respectively, CHO-S cells expressing anti-PD-1 antibody and CHO-S cells expressing Her2 antibody were obtained respectively.
[0036] Specifically, 24 hours before transfection, 0.6×10 6 CHO-S cells were subcultured into freshly prepared CD CHO (Gibco) medium at a cell density of 1 / ml, and placed at 37°C, 8% CO 2 , 130r / min shaker culture. During transfection, preheat the Nucleofector Kit and medium, and prepare 800 μl electrotransfer solution according to the Nucleofector solution:supplement ratio of 4.5:1, add 50 μg plasmid to the electrotransfer solution, and mix well. 1000r / min, 5min centrifugation 3×10 7 cells, discard the supernatan...
Embodiment 3
[0039] Example 3 Respective expression of anti-HER2 antibody and anti-PD-1 antibody
[0040] The seed cells of anti-Her2 antibody and anti-PD-1 antibody were divided into 0.6×10 6 The inoculum density of cells / ml was diluted into a 3L cell culture reactor, and the initial culture volume was 1L; the stirring speed was set to 250r / min; the pH was set to 7.00, DeadBand was set to 0.10, and the associated sodium bicarbonate solution (NaHCO 3 90.7g / L) and CO 2 Adjustment is made; the dissolved oxygen is set to 40%; the culture temperature is set to 36.5° C. for 1 to 4 days; the temperature is lowered to 32.0° C. on the 5th day of cultivation;
[0041] (1) Two cell reactors were fed with feed medium on days 3, 5, 7, 9, and 11. Add feed medium A and feed medium B, and feed medium A The feed volume is 4% of the initial culture volume, and the feed volume of feed medium B is 0.4% of the initial culture volume. Sample 3 mL every day to detect the viable cell density, cell viability a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com