Immune response stimulating composition comprising nanoparticles based on a methyl vinyl ether-maleic acid copolymer
一种甲基乙烯基醚、纳米颗粒的技术,应用在包含基于甲基乙烯基醚-马来酸酐共聚物的纳米颗粒的刺激免疫反应的组合物领域,能够解决免疫原或变应原活性成分剂量高等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] immunity therapy
[0151] Preparation and Characterization of Ovalbumin-Based Methyl Vinyl Ether-Maleic Anhydride (PVM / MA) Copolymer Nanoparticles
[0152] The protein chosen was ovalbumin (OVA) as it is currently widely used as an experimental model for allergies.
[0153] Ovalbumin accounts for more than 50% of the protein content in egg whites. It is a monomeric phosphorylated glycoprotein with a molecular weight of 43 to 45 kDa and 385 amino acids [Johnsen and Elsayed, Mol Immunol, 27 (1990) 821]. Ovalbumin is the egg protein with the strongest allergenic ability, which immediately induces a type I hypersensitivity reaction mediated by IgE.
[0154] The methods described below can be used to efficiently prepare nanoparticle-based colloidal dosage forms for immunotherapy.
[0155] 1.1 Preparation of empty nanoparticles (NP)
[0156] 100 mg of methyl vinyl ether-maleic anhydride copolymer (PVM / MA) [Gantrez_AN 119] was dissolved in 5 mL of acetone. Next, 10 mL of ...
Embodiment 2
[0192] In vitro testing of release of ovalbumin from nanoparticles
[0193] To assess the release of ovalbumin in vitro, nanoparticles were incubated in 1 mL of PBS (phosphate buffered saline, pH 7.4) at a concentration of approximately 8 mg / mL in "eppendorf" tubes. Tubes were incubated in an oven at 37 °C under rotation, and samples were centrifuged at 26,500 x g for 20 min at predetermined time intervals, and supernatants were collected for subsequent analysis. The released ovalbumin was detected by the bicinchoninic acid method.
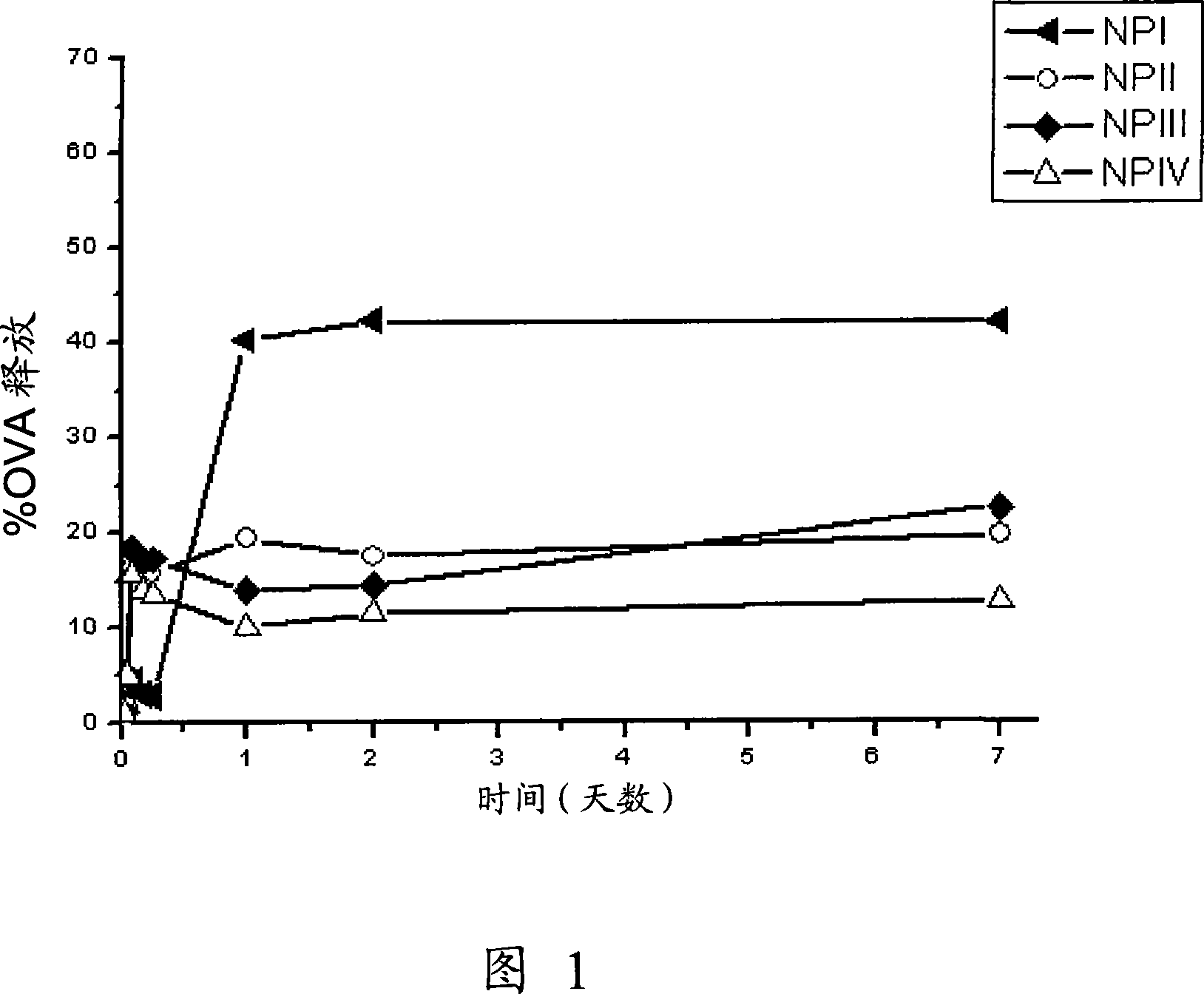
[0194] The obtained release profile of ovalbumin is represented in Figure 1; it was observed that ovalbumin was faster in formulation NP I than in formulations NP II, NP III and NP IV which exhibited similar release profiles. This is because in formulation NP I, ovalbumin is adsorbed on the outer surface of the nanoparticles, so the initial release (burst) is slower than in other formulations (NP II, III and IV) where ovalbumin is partially encap...
Embodiment 3
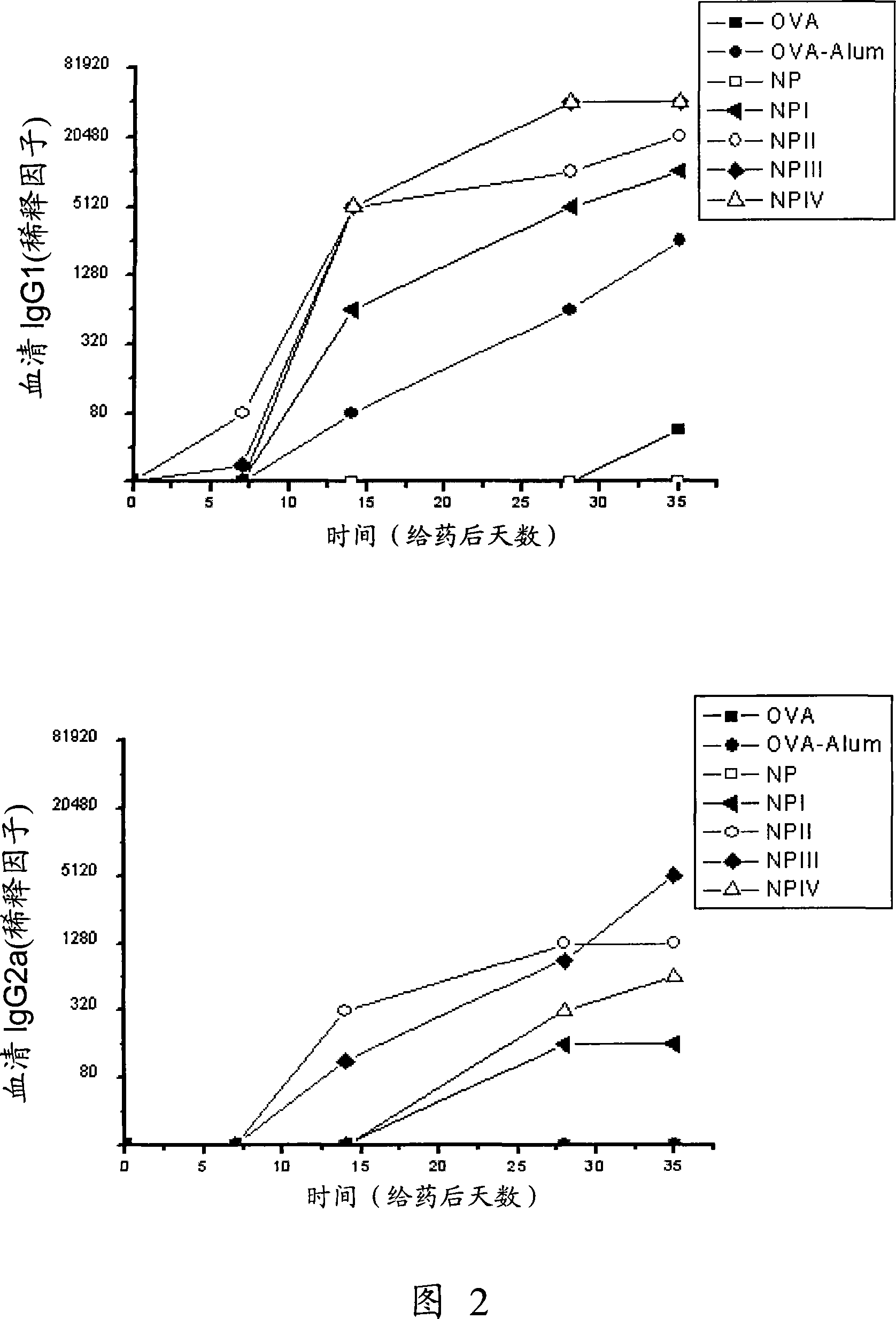
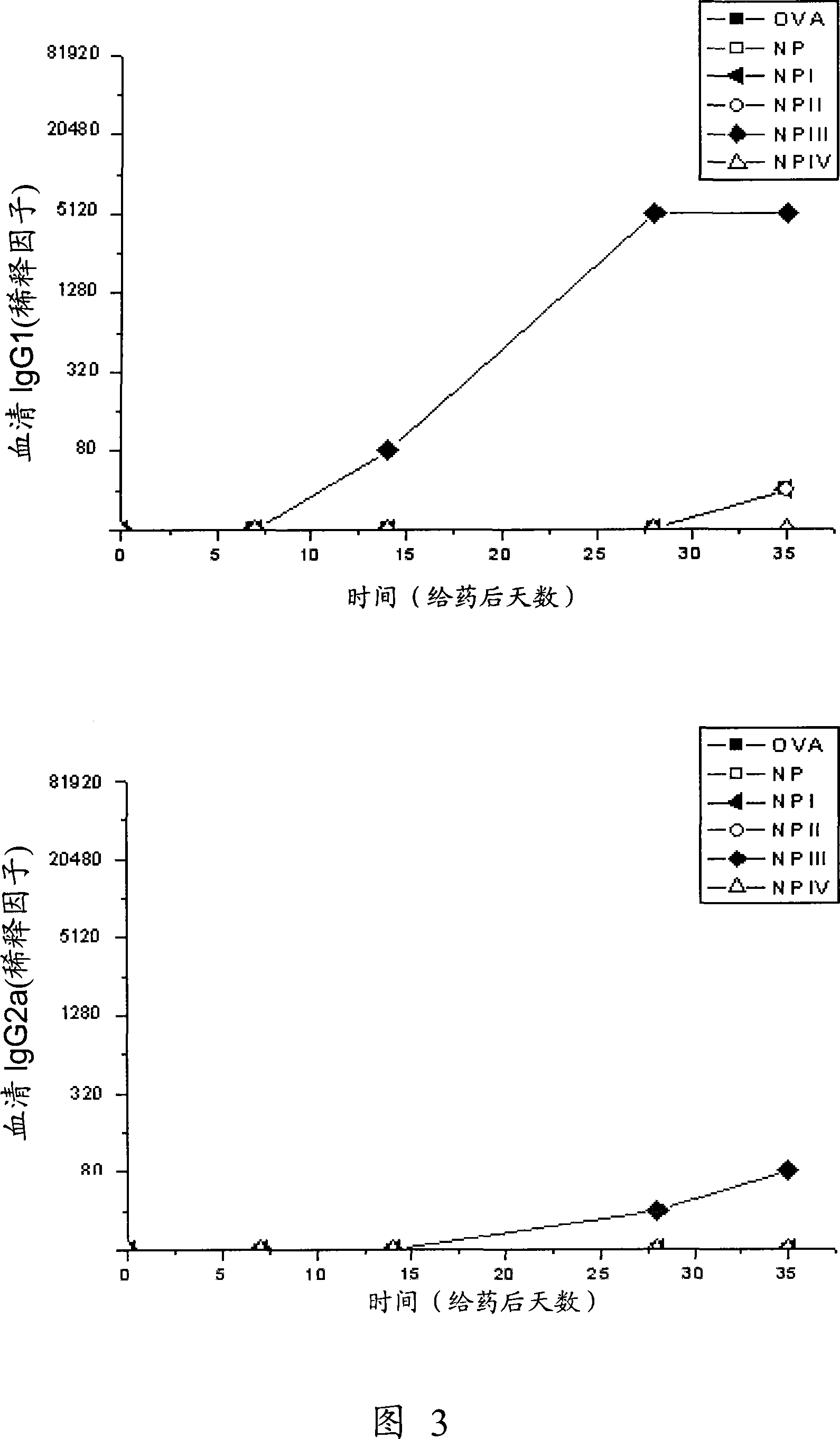
[0196] Quantification of anti-OVA antibodies produced after immunization with ovalbumin in BALB / c mice
[0197] 65 BALB / c mice were immunized, and they were divided into 13 groups according to the method of administration.
[0198] The controls used were ovalbumin-free solution (OVA) (10 μg intradermally, 25 μg orally) and empty nanoparticles (NP) (intradermally and orally), as well as ovalbumin adsorbed to aluminum hydrogel. Albumin (OVA-Alum) (10 μg administered intradermally) served as a positive control for inducing a Th2 response characterized by high IgG 1 Potency [Faquim-Mauro et al., Int Immunol, 12 (2000) 1733-1740].
[0199] The remaining groups were vaccinated intradermally (10 μg OVA) or orally (25 μg OVA), and the treatments were:
[0200] a) intradermal inoculation of ovalbumin (OVA) solution
[0201] b) Oral inoculation with ovalbumin (OVA) solution
[0202] c) Intradermal inoculation of ovalbumin (OVA-Alum) adsorbed on aluminum hydrogel
[0203] d) Intrade...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap