Method and device for reactivating inactivated nickel for carbonyl nickel production
A carbonyl nickel and reactivation technology, applied in the direction of carbonyl nickel, carbonyl iron, etc., can solve the problems of not providing an easy and convenient reactivation method, easy deactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
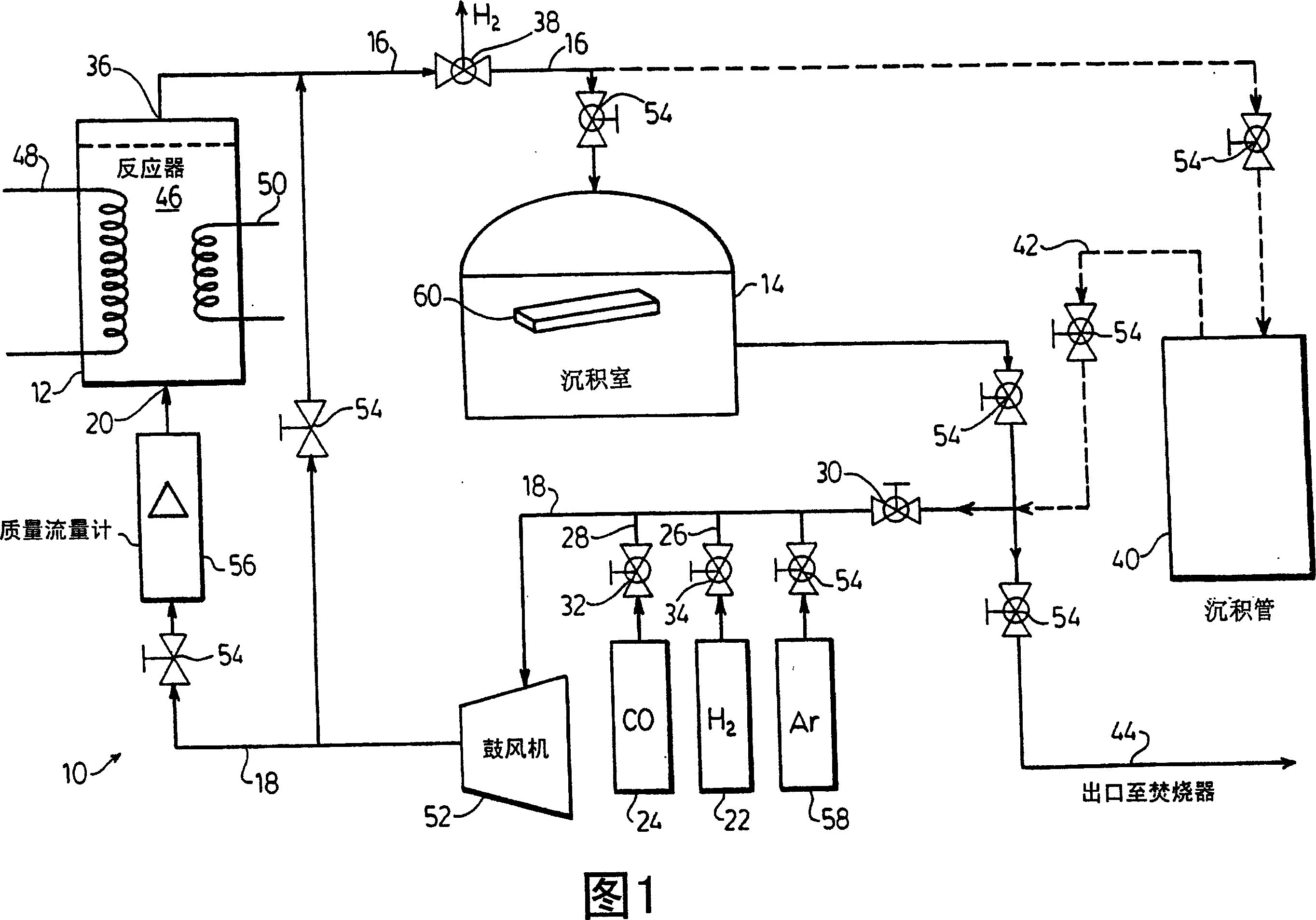
[0049] Referring to FIG. 1, there is shown a closed loop apparatus, shown generally at 10, comprising a reactor 12 connected by conduits 16 and 18 to a decomposition chamber 14.
[0050] The reactor 12 has an inlet conduit 20 through which hydrogen and carbon monoxide are alternately fed into the reactor 12 as required. As will be described hereinafter, conduit 18 has connections to a source of hydrogen 22 and an initial source of carbon monoxide 24 and leads to inlet conduit 20 via conduits 26, 28, respectively, as required. Conduit 18 has a selector valve 30 and an initial source valve 32 for controlling the passage of carbon monoxide from chamber 14 or initial source 24 to inlet conduit 20 as desired. Hydrogen is passed through line 18 to inlet line 20 since the initial activation reaction is controlled by selector valve 34 .
[0051] Reactor 12 has an outlet conduit 36 through which initially the spent hydrogen gas is discharged to a portion of conduit 16 or the spent c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com