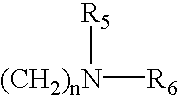A1 adenosine receptor antagonists
a technology of adenosine receptor and antagonist, which is applied in the field of a1 adenosine receptor antagonist, can solve the problems of poor water solubility, low potency or lack of selectivity of adenosine receptor, and the development of adenosine receptors has not fully addressed the problems of potency and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Synthesis of A.sub.1 Adenosine Receptor Antagonists
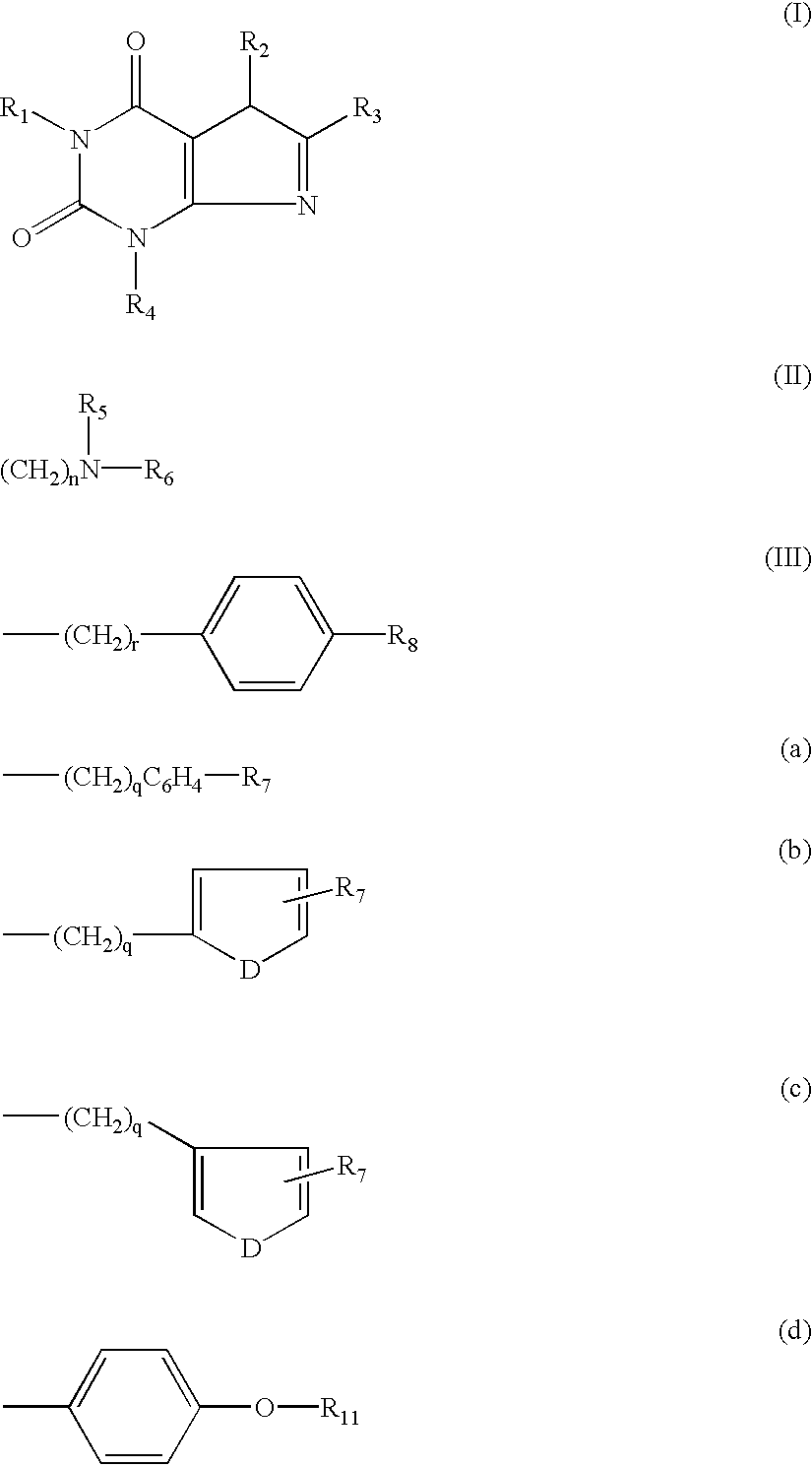
[0045] A.sub.1 adenosine receptor antagonists of the present invention may be synthesized according to the process illustrated below: 10
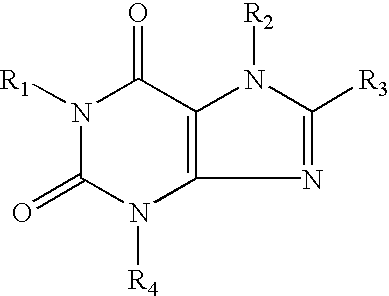
[0046] In the above reaction pathway, R' may be C.sub.1-C.sub.8 alkyl; R" may be selected from the group consisting of H, OH, (CH.sub.3).sub.eNO.sub.2 wherein e is selected from the group consisting of 0 and an integer ranging from 1 to 8; (CH.sub.2).sub.sOH, wherein s is an integer ranging from 1 to 8; and R.sub.10COOH, wherein R.sub.10 is an alkylene or alkenylene group having 1 to 8 carbon atom; R.sup.x may be selected from the group consisting of:
(CH.sub.2).sub.qC.sub.6H.sub.4R'" 11
[0047] wherein R'" may be selected from the group consisting of H, OH, NO.sub.2, R.sub.9COOH, wherein R.sub.9 is an alkylene or alkenylene group having 1 to 8 carbon atoms, and (CH.sub.2).sub.tOH, wherein t is an integer ranging from 1 to 8; D may be selected from the group consisting of O, S, and NH; q is an integer ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| non-radioactive | aaaaa | aaaaa |
| fluorescent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com