Drug delivery compositions and methods
a technology of compositions and drugs, applied in the field of drug delivery compositions, can solve problems such as complex problems, inadequate cell proliferation, and potentially catastrophic consequences of infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
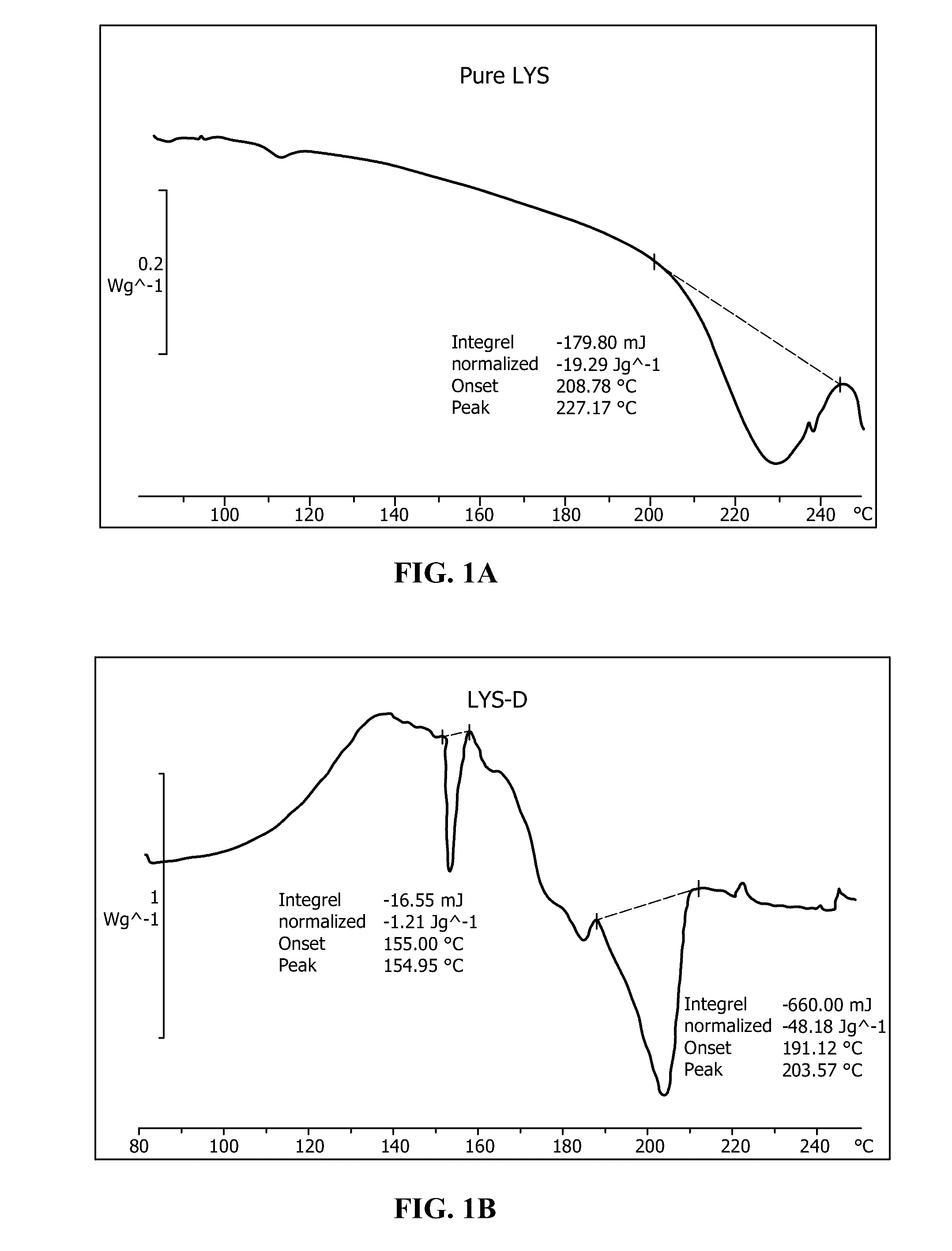
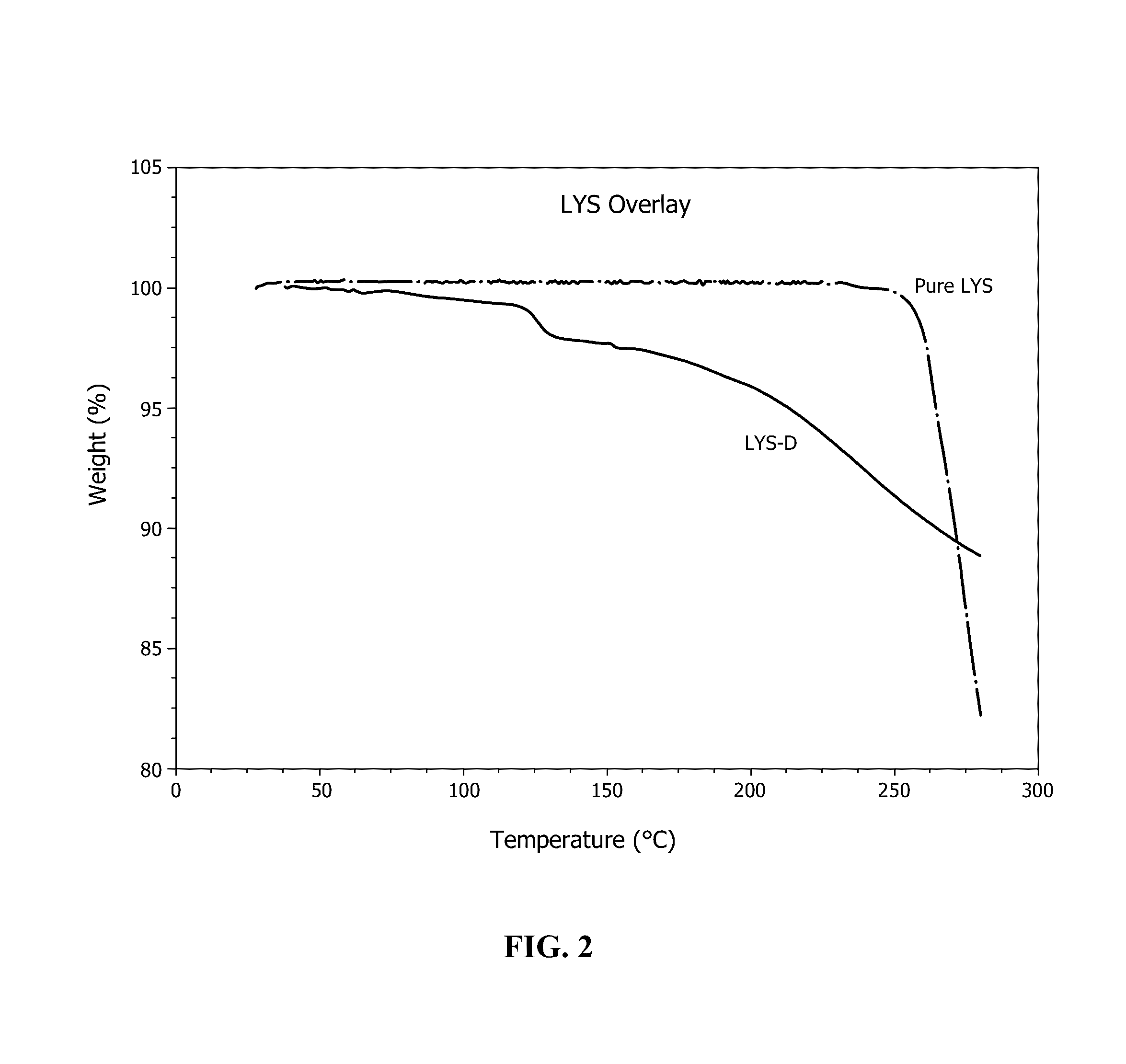
[0032]150 grams of potassium peroxymonosulfate triple salt were mixed with 5 g Lysine amino acid. This mixture was reacted with 100 g water in order to preferentially dissolve the K+HSO5− component out of the triple salt with immediate reaction with the amino acid, forming a slurry. This slurry was mechanically stirred in a glass beaker for a period of one hour. After one hour, the slurry mixture was vacuum filtered, and the resulting liquid material was collected and crystallized at room temperature. The crystallized powder materials were air dried to yield a dry powder base material. The powder base material was collected in a resealable plastic bag, and stored in a plastic bucket with anhydrous calcium sulfate desiccant material (Drierite®, W. A. Hammond Drierite Co. Ltd., Xenia, Ohio) placed in the bottom of the bucket. The bucket was closed with an air-tight lid. The activity of the triple salt proxy functional group decreased by less than 0.1% per month over the course of a ye...
example 2
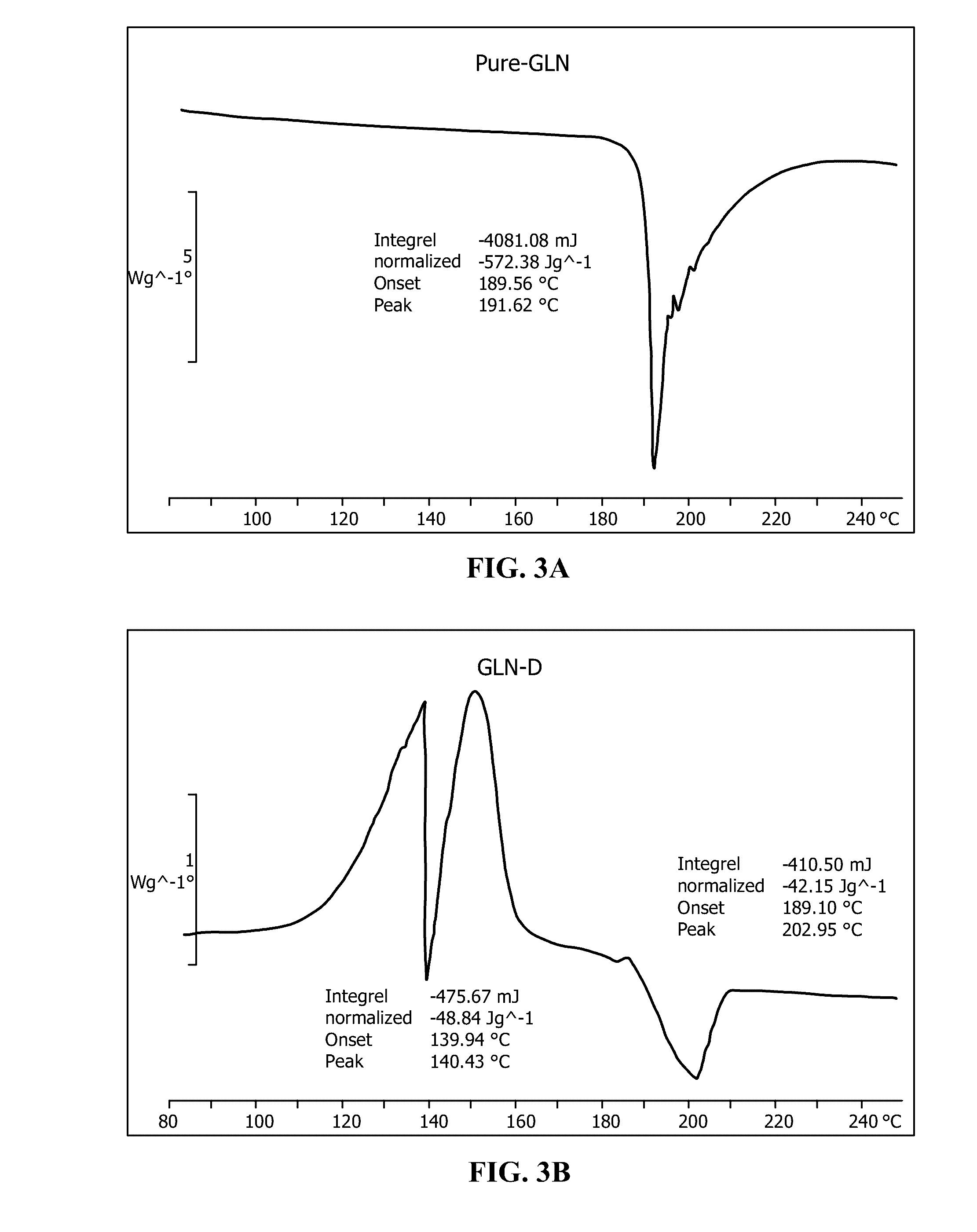
[0034]150 grams of potassium peroxymonosulfate triple salt were mixed with 5 g Glutamine amino acid. This mixture was reacted with 100 g water in order to preferentially dissolve the K+HSO5− component out of the triple salt with immediate reaction with the amino acid, forming a slurry. This slurry was mechanically stirred in a glass beaker for a period of one hour. After one hour, the slurry mixture was vacuum filtered, and the resulting liquid material was collected and freeze dried to yield a powder base material. The powder base material was collected in a resealable plastic bag, and stored in a plastic bucket with anhydrous calcium sulfate desiccant material (Drierite®, W. A. Hammond Drierite Co. Ltd., Xenia, Ohio) placed in the bottom of the bucket. The bucket was closed with an air-tight lid. The activity of the triple salt proxy functional group decreased by less than 0.1% per month over the course of a year.
[0035]Referring to FIGS. 3A and B, differential scanning calorimetri...
example 3
[0036]150 grams of peroxymonosulfate triple salt were reacted with 100 g water in order to preferentially dissolve the K+HSO5− component out of the triple salt, forming a slurry. This slurry was mechanically stirred in a glass beaker for a period of one hour. After one hour, the slurry mixture was vacuum filtered, and the resulting liquid was reacted with 5 g Glycine amino acid. The reaction product was collected and freeze dried to yield a powder base material. The powder base material was collected in a resealable plastic bag, and stored in a plastic bucket with anhydrous calcium sulfate desiccant material (Drierite®, W. A. Hammond Drierite Co. Ltd., Xenia, Ohio) placed in the bottom of the bucket. The bucket was closed with an air-tight lid. The activity of the triple salt proxy functional group decreased by less than 0.01% per month over the course of a year.
Example 4
[0037]44 grams of peroxymonosulfate triple salt were reacted with 100 g water in order to preferentially dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| onset decomposition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com