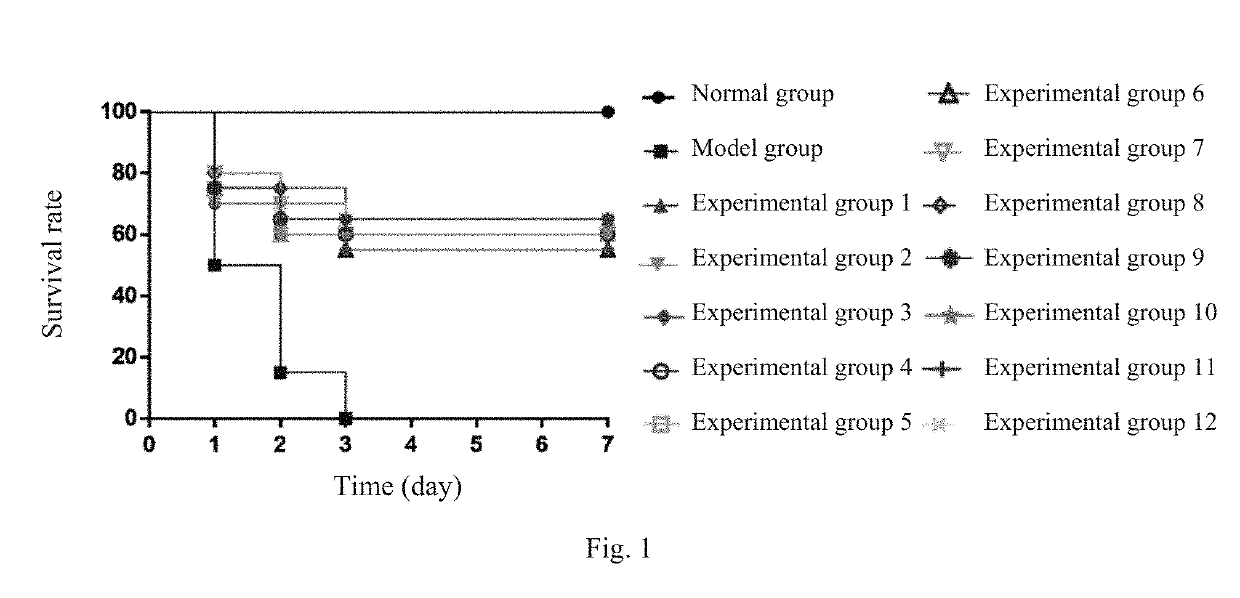
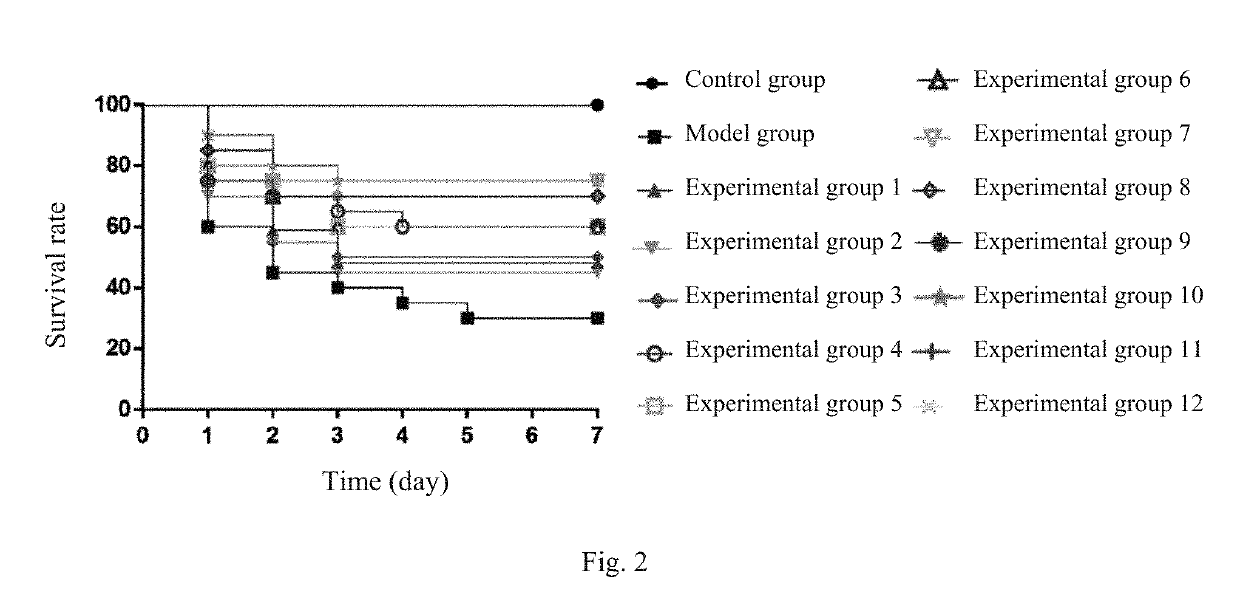
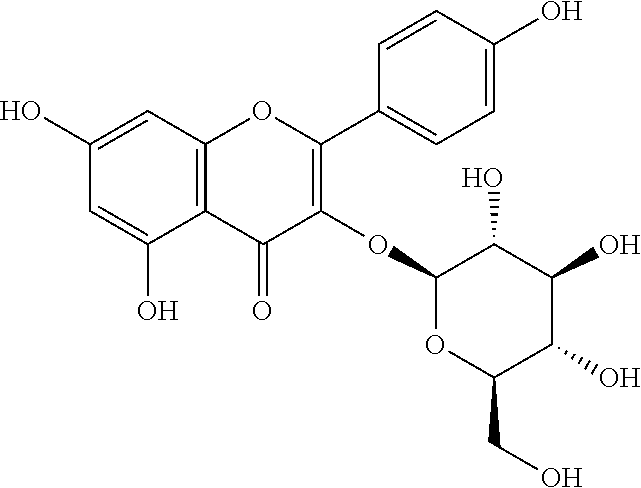
A multi-component injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Embodiment 1 Testing Method for Effective Ingredients in Injection
[0103]Method 1: Chromatographic Separation Conditions for Analyzing Ingredients of Safflower Carthamus and Red Paeony Root
[0104]Chromatographic column: Waters HSS T3 UPLC C18 Chromatographic column (100 mm×2.1 mm; 1.8 μM, Waters, USA);
[0105]Column temperature: 45° C.;
[0106]Mobile phase: A: H2O (containing 25 mM HCOOH, B: MeOH (containing 25 mM HCOOH);
[0107]Gradient elute is shown in Table 6; flow velocity: 0.35 mL / min; injection volume: 5 μL; analysis time: 13 min.
TABLE 6Liquid-phase gradient elute conditions of ingredients of safflowercarthamus and Red Paeony RootTime (min)Solvent ASolvent B0.098%2%8.030%70%11.02%98%13.098%2%
[0108]Method 2: Chromatographic Separation Conditions for Analyzing Ingredients of Radix Salviae Miltiorrhizae
[0109]Chromatographic column: Waters HSS T3 UPLC C18 Chromatographic column (100 mm×2.1 mm; 1.8 μM, Waters, USA);
[0110]Column temperature: 45° C.;
[0111]Mobile phase: A: H2O (containing 25...
Example
Embodiment 2 Preparation of Multi-Component Injection
[0117]providing 100 g of Red Paeony Root decoction pieces; heating and boiling the Red Paeony Root decoction pieces with process water of 10 times the weight of the Red Paeony Root decoction pieces to obtain a first decoction after 2 hours slight boiling; filtering the first decoction to obtain filtrate I and a first dreg; boiling the first dreg with process water of 8 times the weight of the first dreg to obtain a second decoction after 1 hour slight boiling; filtering the second decoction to obtain filtrate II and a second dreg; mixing filtrate I and filtrate II to obtain a mixture; concentrating the mixture to obtain a first concentrate of 100 ml; adding a proper amount of gelatin solution to the first concentrate under stirring to obtain a gelatin-containing first concentrate; adding 95% ethanol to the gelatin-containing first concentrate to obtain an ethanol-containing first concentrate having an ethanol volume content of 70%...
Example
Embodiment 3 Preparation Process of Multi-Component Injection
[0120]providing 100 g of safflower carthamus decoction pieces; leaching the safflower carthamus decoction pieces with 30% ethanol of 8 times the weight of the safflower carthamus decoction pieces for 8 hours to obtain a leach liquor; filtering the leach liquor to obtain a liquid medicine of 4-6 times the weight of the safflower carthamus decoction pieces; adding 95% ethanol to the liquid medicine to obtain an ethanol-containing liquid medicine having an ethanol volume content of 70%; storing the ethanol-containing liquid medicine under cooling condition for 48 hours to obtain a cool ethanol-containing liquid medicine; filtering the cool ethanol-containing liquid medicine to obtain a first filtrate; concentrating the first filtrate under reduced pressure to obtain a concentrate of 100 ml; adding 95% ethanol to the concentrate to obtain an ethanol-containing concentrate having an ethanol volume content of 80%; storing the et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap