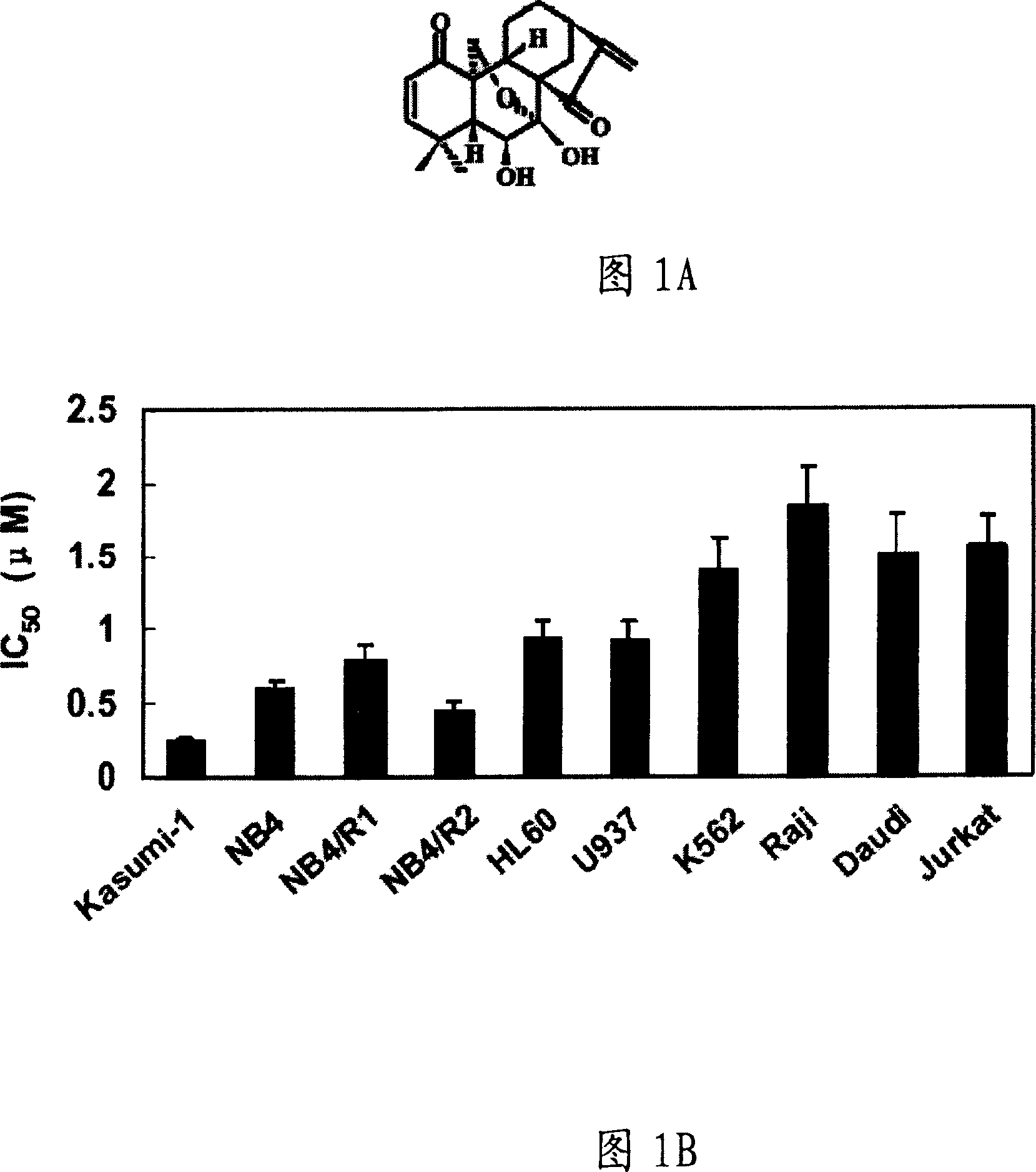
Application of Maoeryisu for pharmacy
一种毛萼乙素、药物的技术,应用在毛萼乙素领域,能够解决不能治疗急性白血病等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] EriB inhibits growth and induces apoptosis in human leukemia cells
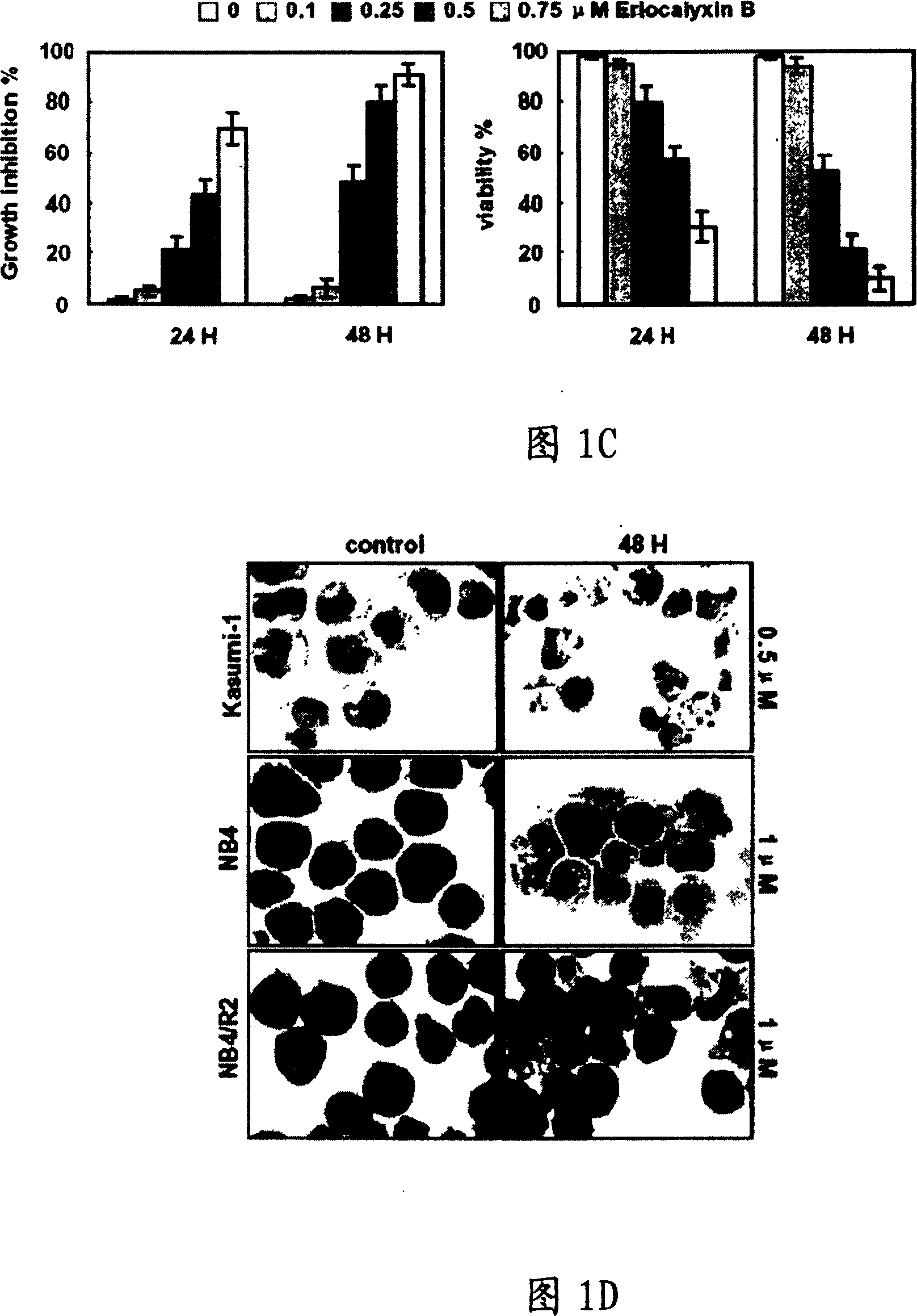
[0074] The effect of EriB on the growth of human hematologic malignancies was detected by MTT assay. The half-inhibition of growth of these cells by EriB was between 0.2-2 μM. Different concentrations of EriB have growth inhibitory effect on all tested cells. The half-inhibitory dose of EriB on Kasumi-1 cells was 0.2 μM, on NB4 and NB4 / R2 cells was 0.5 μM, and on NB4R1, HL60 and U937 cells was 1 μM. Malignant lymphoproliferative disorders are less sensitive to eillin than myeloid leukemia cells, IG of Raji, Daudi and Jurkat cells 50 Values above 1.5 μM (see Figure 1B).
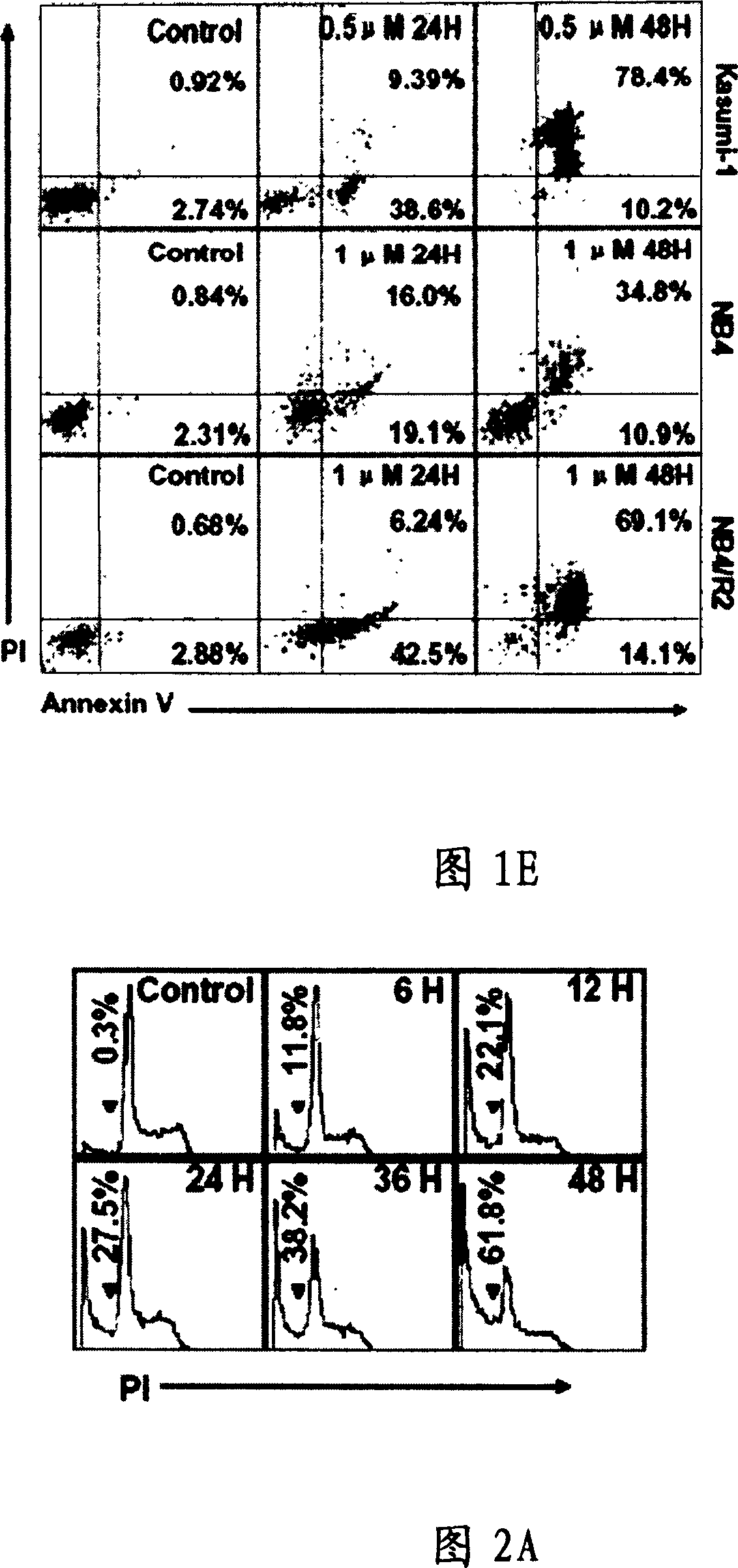
[0075] Kasumi-1 cells are the most sensitive to EriB, and EriB has a time- and dose-dependent inhibitory effect on cell growth (see Figure 1C left), and the cell survival rate also decreases correspondingly (see Figure 1C right). Inhibitory effects were also observed at concentrations below 0.25 μM. After Kasumi-1 cells were tre...
Embodiment 2
[0079] Elein B disrupts mitochondria and activates caspase-3 at apoptosis-inducing concentrations
[0080] In order to study the ultrastructural changes of Kasumi-1 cells in the early stage of apoptosis and to search for hints of mechanisms involved in apoptosis, we performed transmission microscopy analysis. Normal tumor cell nuclei were irregular in shape and contained a large number of rough endoplasmic reticulum and mitochondria (see Figure 2B), and Kasumi-1 cells treated with EriB for 6 hours showed swollen cells, increased number of lysosomes, swelling and Weakened mitochondria (see Figure 2B), suggesting early apoptosis and mitochondrial damage (see Figure 2B).
[0081] Kasumi-1 cells treated with EriB were incubated with Rhl23 and then used for flow cytometry detection. The results showed that the mitochondrial transmembrane potential decreased significantly, and there was a time and dose dependence (see FIG. 2C ). In Kasumi-1, NB4 and NB4 / R2 cells, the cleavage of ca...
Embodiment 3
[0083] Bc1-2 and Bc1-X via down-regulation of Bc1-X L Acts on intrinsic apoptotic pathway
[0084]Bcl-2 family proteins directly control the permeability of mitochondrial membranes, and they are central regulators of caspase activation. The relative members of this family, anti-apoptotic and pro-apoptotic, determine the fate of cells. In order to detect whether EriB damages mitochondria by acting on Bc1-2 family members, we used western blot and RT-PCR to detect the anti-apoptotic factors Bc1-2 and Bc1 in Kasumi-1 cells treated with EriB for 24 and 48 hours -X L And the expression of pro-apoptotic factor Bax. Bc1-2 and Bc1-X L There was a reduction at both protein and mRNA levels, however Bax expression did not change significantly (see Figure 3A and Figure 3B). Finally, EriB induces Bc1-2 / Bax and Bc1-X L Negative regulation of the / Bax ratio (see Figure 3C and Figure 3D), which is consistent with the results of disrupted mitochondrial stability during apoptosis in Kasum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com