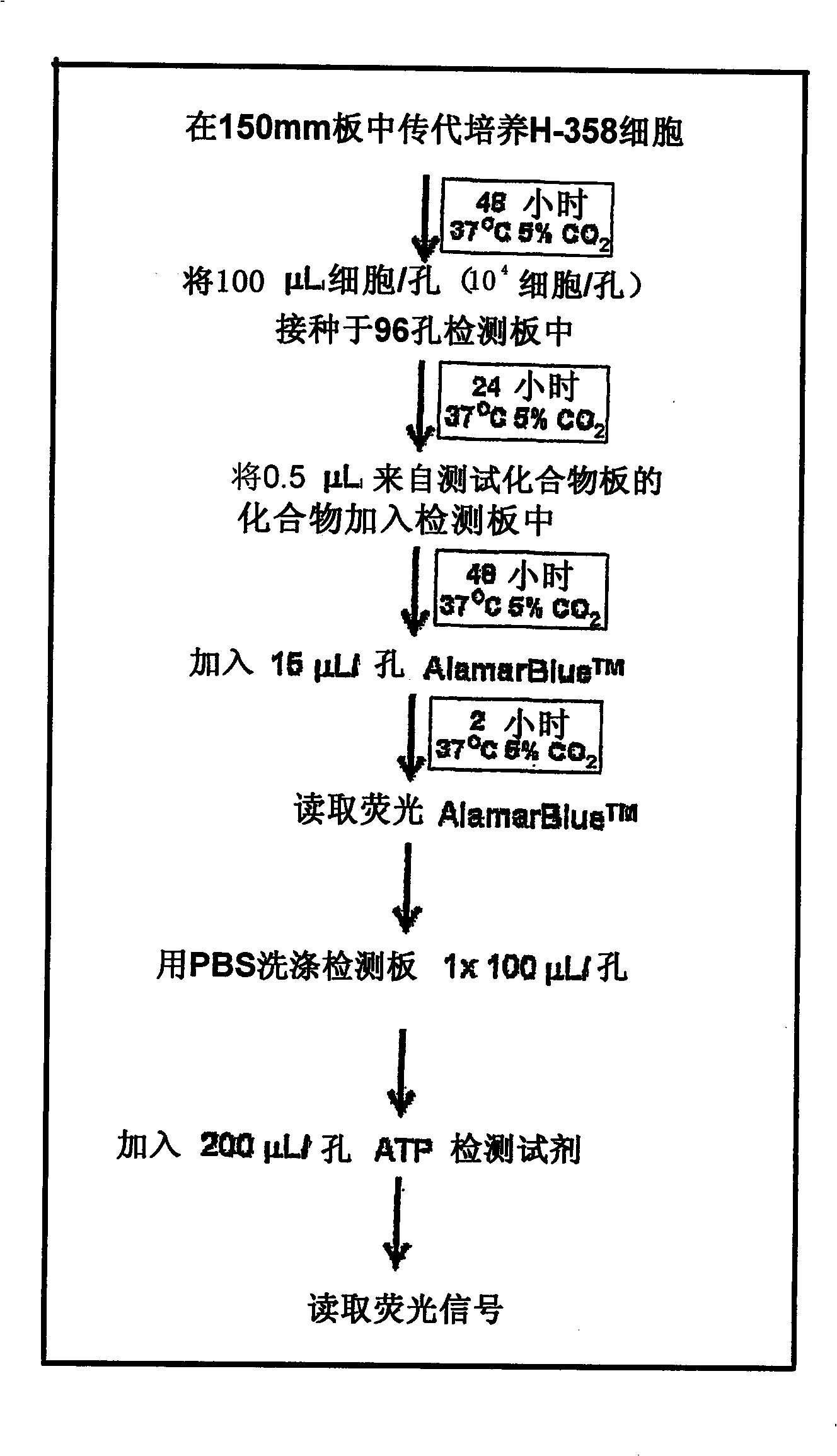
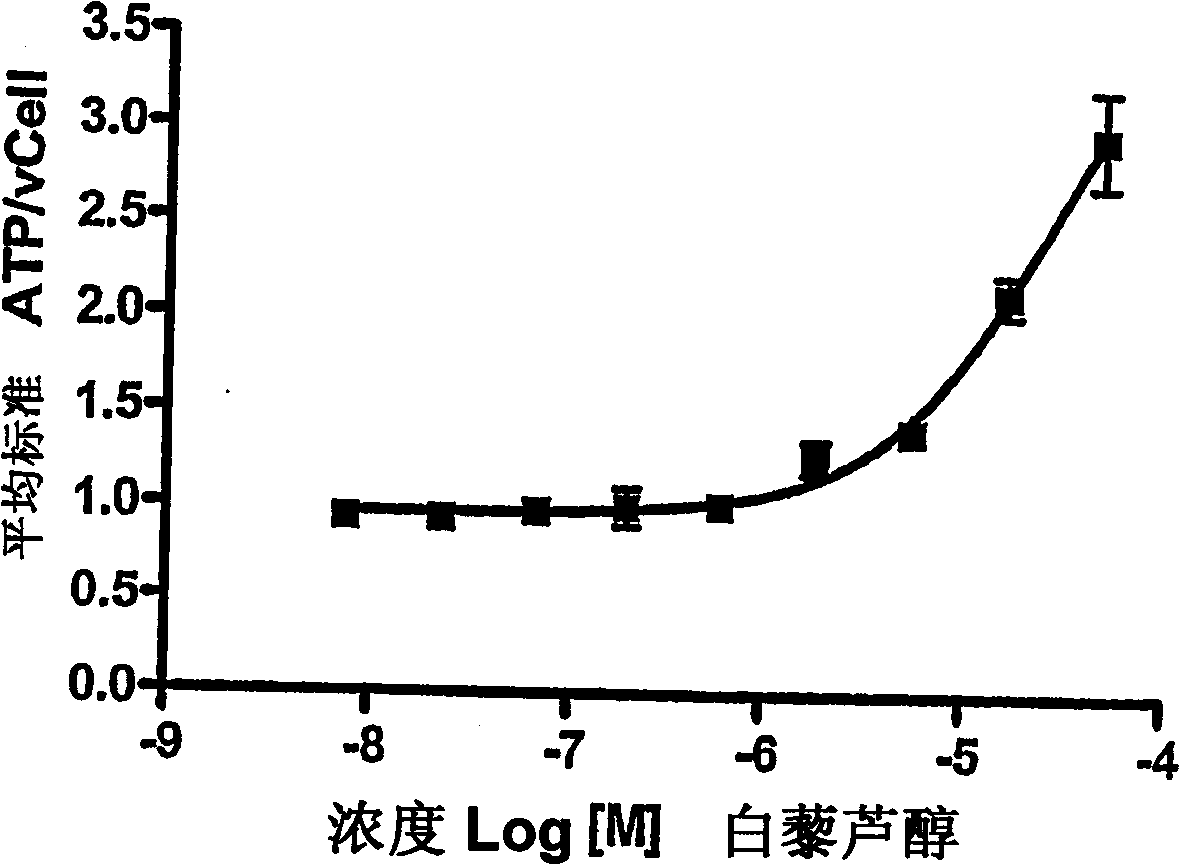
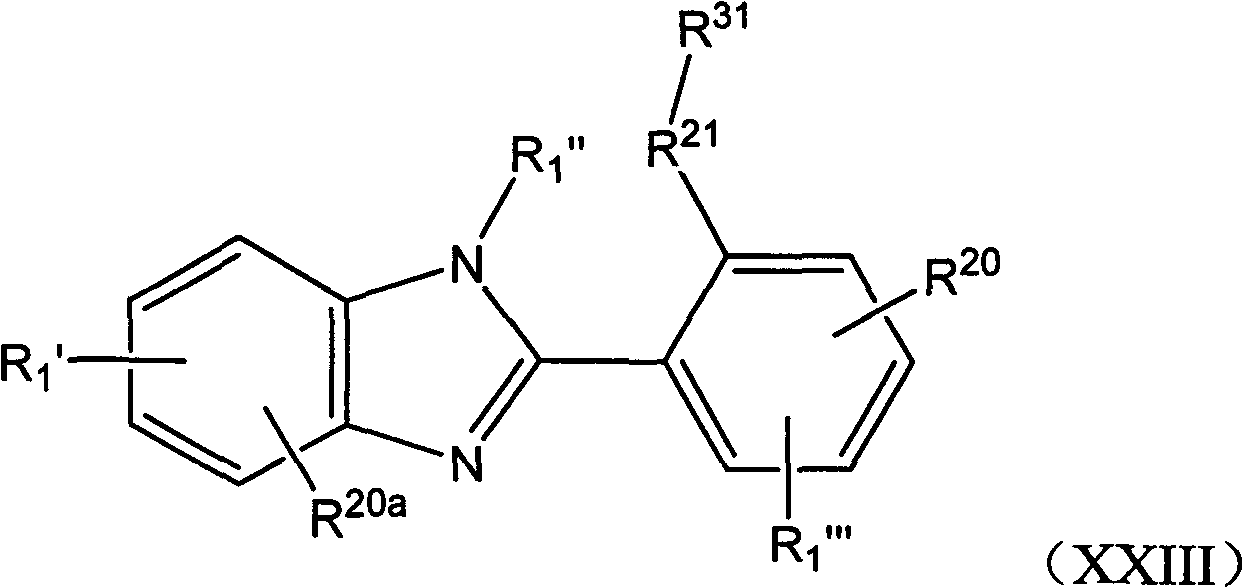
Benzimidazole derivatives as SIRTUIN modulators
A phenyl and compound technology, applied in the field of benzimidazole derivatives as SIRTUIN regulators, can solve problems such as the inability to prolong the lifespan of mutant sir2 strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1691] Synthesis and characterization of embodiment 1 Sirtuin modulator
[1692] General route:
[1693] Route 1:
[1694]
[1695] Route 2:
[1696]
[1697] Route 3:
[1698]
[1699] Route 4:
[1700]
[1701] Experimental part:
[1702] Abbreviations used in the experimental part:
[1703] HATU=O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate
[1704] NMM=4-methylmorpholine
[1705] DIEA=N,N-Diisopropylethylamine
[1706] DMF=N,N-Dimethylformamide
[1707] CH 2 Cl 2 = dichloromethane
[1708] EtOAc = ethyl acetate
[1709] MeOH = Methanol
[1710] Na 2 SO 4 = sodium sulfate
[1711] PPA = polyphosphoric acid
[1712] Et 3 N = triethylamine
[1713] rt = room temperature
[1714] Preparation of 3-(thiazolo[5,4-c]pyridin-2-yl)aniline:
[1715]
[1716] 4-aminopyridin-3-yldiisopropylcarbamoylcarbamodithioate (4-aminopyridin-3-yldiisopropylcarbamodthioate) according to Smith et al, Sulfur Lett.1994 vol 17, p.197 an...
Embodiment 2
[2459] Example 2: Identification of Sirtuin Modulators
[2460] The activity of SIRT1 modulators is identified using fluorescence polarization or mass spectrometry based assays. The same assay can be used to identify modulators of any sirtuin protein. The fluorescence polarization assay uses one of two different peptides based on the known target of sirtuin deacetylation - the p53 fragment. Compounds 1-18 were detected with a substrate containing peptide 1 having the following 14 amino acid residues: GQSTSSHSK(Ac)NleSTEG (SEQ ID NO: 1), where K(Ac) is an acetylated lysine residue, Nle For norleucine. The peptide was labeled C-terminally with the fluorophore MR121 (excitation 635 nm / emission 680 nm) and N-terminally with biotin. The sequences of the peptide substrates are based on p53 with various modifications. In particular, unlike acetylated lysines, all arginine and leucine residues are replaced with serines, rendering the peptide less susceptible to cleavage by trypsin...
Embodiment 3
[2596] Example 3: Identification of Sirtuin Modulators Using SIRT3
[2597] Identification of SIRT3 modulator activity by fluorescence polarization assay. Any modulator of a sirtuin protein can be identified using the same assay. The assay utilizes a peptide substrate based on a known sirtuin deacetylation target, the histone H4 fragment. The substrate contained a peptide with the following 14 amino acid residues: Biotin-GASSSHSK(Ac)VLK(MR121) (SEQ ID NO: 4), where K(Ac) is an acetylated lysine residue. The peptide was labeled C-terminally with the fluorophore MR121 (excitation 635 nm / emission 680 nm) and N-terminally with biotin.
[2598] Peptide substrates in NAD + Exposure to the presence of sirtuin proteins deacetylates the substrate, rendering it susceptible to cleavage by trypsin. Then trypsin was added, and the reaction was carried out to completion (eg, the deacetylated substrate was cleaved), releasing the MR121 fragment. Streptavidin, which binds the uncleaved s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com