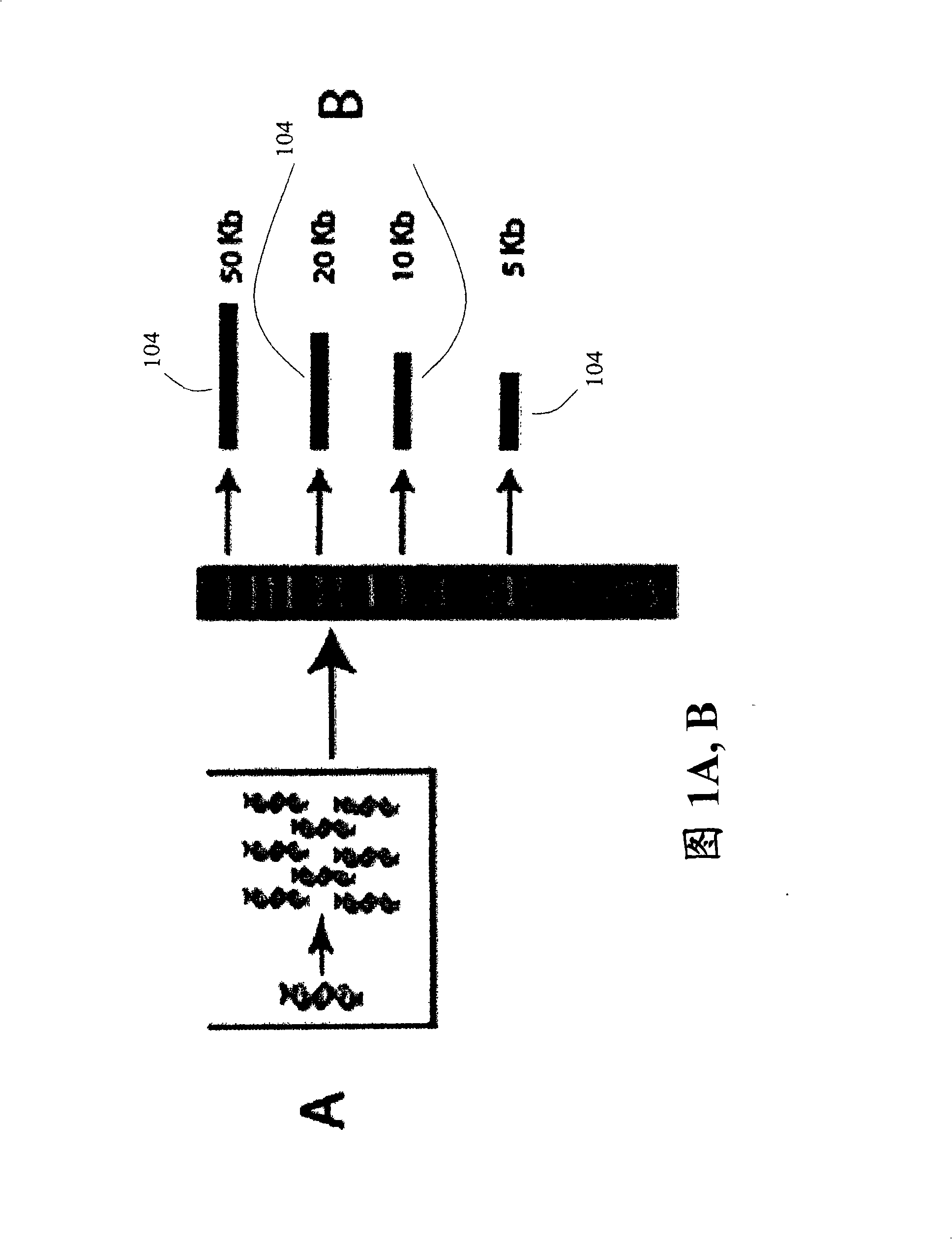
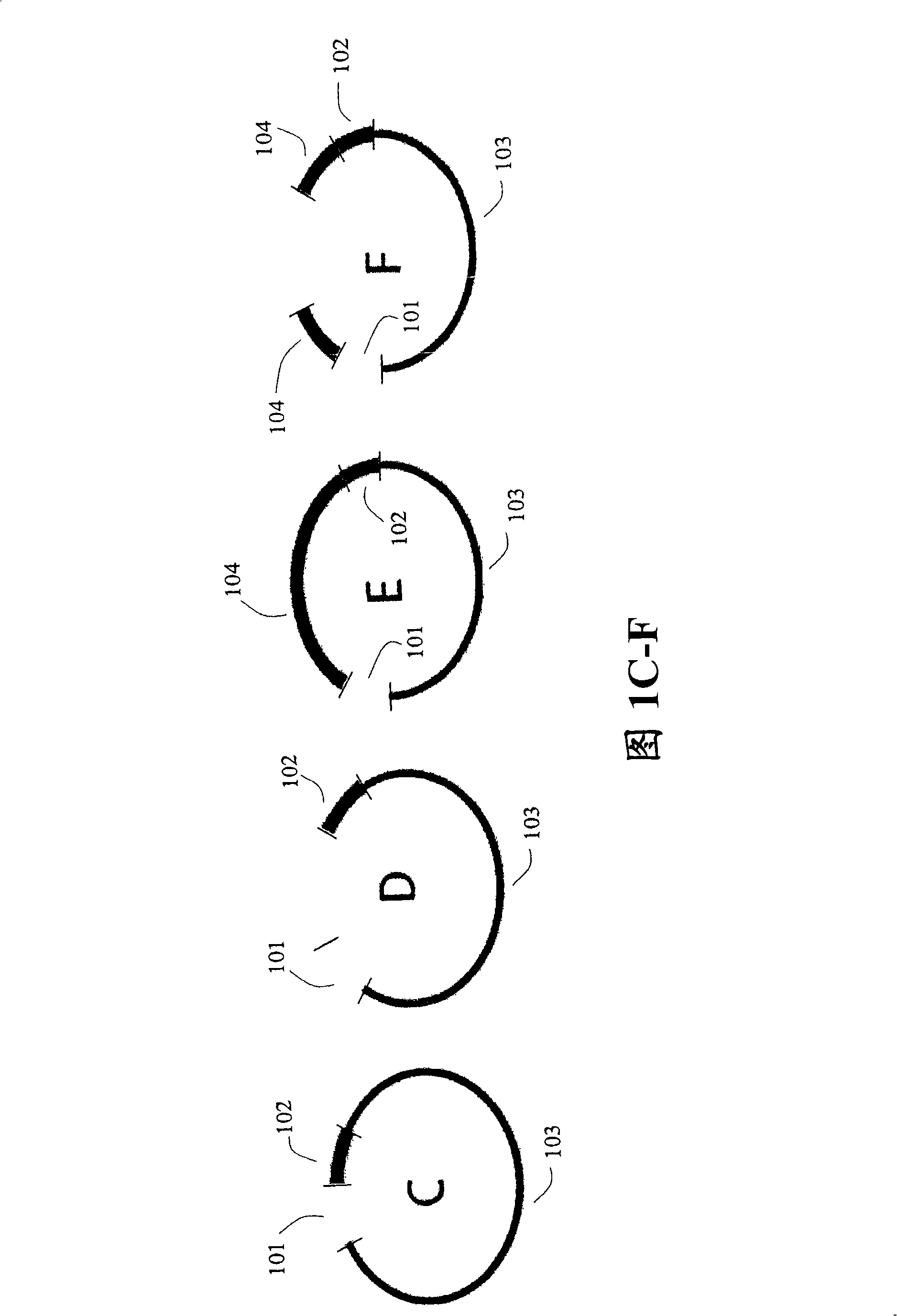
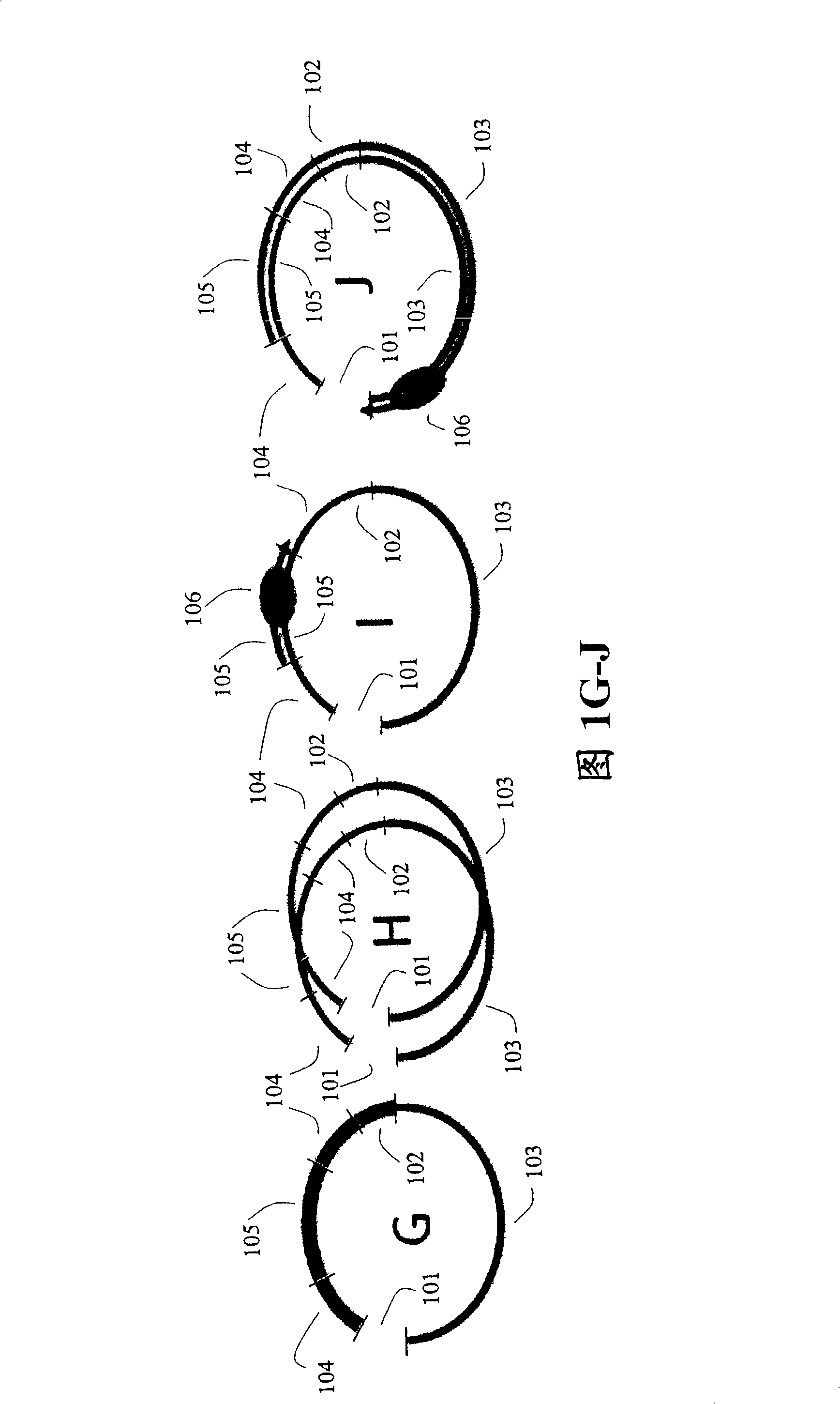
Paired end sequencing
A nucleic acid and target technology, applied in the field of paired-end sequencing, can solve the problems of difficult and time-consuming sequencing of large nucleic acid fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0120] Step 2E - Preparation of Fragments for Circularization
[0121] After adding adapters to both ends of the nucleic acid fragment of interest, the fragment is circularized.
[0122] To prepare target nucleic acids for self-circularization, cleavage in the linker region may be desirable for various reasons. For example, if a hairpin ligation is used, the DNA fragments will not self-circularize because there are no free 5' or 3' ends. As another example, if the ligation leaves blunt ends to the DNA fragments, cleavage will give the ligation a 5' or 3' overhang, these overhangs (so called "sticky ends") are very favorable for ligation efficiency. Also, digestion in the adapter region should allow selection of DNA fragments with two adapters, 1 adapter attached to each end. This is because the adapters are digestible such that cleavage with restriction endonucleases leaves matching cohesive ends. After cleavage in the linker region, the DNA fragment with only 1 linker (u...
Embodiment 1
[0272] Example 1: Oligonucleotide Design
[0273] Oligonucleotides used in the experiments were designed and synthesized as follows.
[0274] The capture element oligonucleotides shown at the top of Figure 3A were designed to contain the UA3 linker and key sequences. A NotI site is located between the linkers. Complete constructs (capture elements) can be created using nested oligonucleotides and PCR. Synthesize and clone the final product sequence.
[0275] The Type IIS capture fragment oligonucleotide shown at the bottom of Figure 3A is similar to the capture fragment described above, except that a sequence representing a Type IIS restriction endonuclease site (eg, MmeI) is included in the capture fragment after the key sequence. These Type IIS restriction endonuclease cleavage sites allow cleavage with Type IIS restriction endonucleases of any construct made from these capture elements to be excised. As is known in the art, the IIS restriction endonuclease cleaves DNA...
Embodiment 2
[0277] Example 2: Protocol for paired-end sequencing of hairpin junctions
[0278] A 100 μl solution of E. coli K12 DNA (20 μg) was subjected to 20 cycles of hydroshearing at speed 10 using a standard HydroShear apparatus (Genomic Solutions, Ann Arbor, Mich., USA). By adding 50 μl DNA (5 μg), 34.75 μl H 2 O, 10 μl of methylase buffer, 0.25 μl of 32mM SAM and 5 μl of EcoRI methylase (40,000 units / ml, New England Biolabs (NEB), Ipswich, Mass., USA) carried out methylation on the DNA that had been sheared. reaction. Reactions were incubated at 37°C for 30 minutes. After the methylation reaction, the sheared methylated DNA was purified using Qiagen MinElute PCR purification columns according to the manufacturer's instructions. The purified DNA was eluted from the column with 10 μl of EB buffer.
[0279] Sheared methylated DNA is trimmed to produce sheared material with blunt ends. Add 10 μl DNA to the reaction mixture containing 13 μl HO 2 O. 5 μl 10× polishing buffer, 5 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com