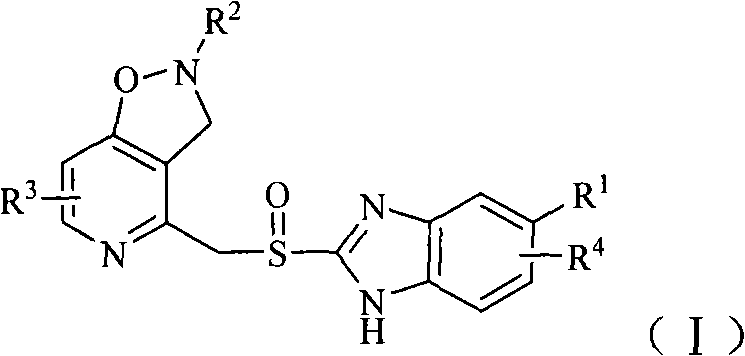
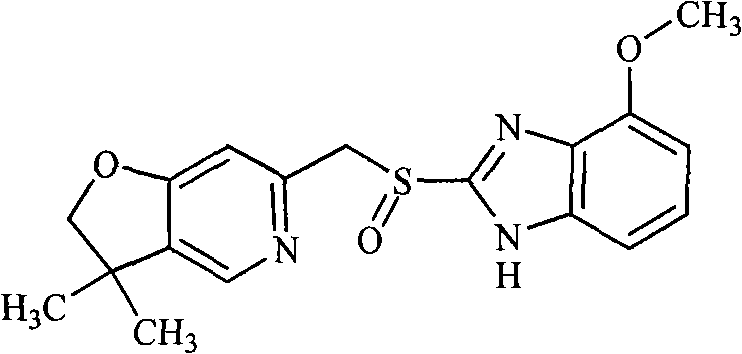
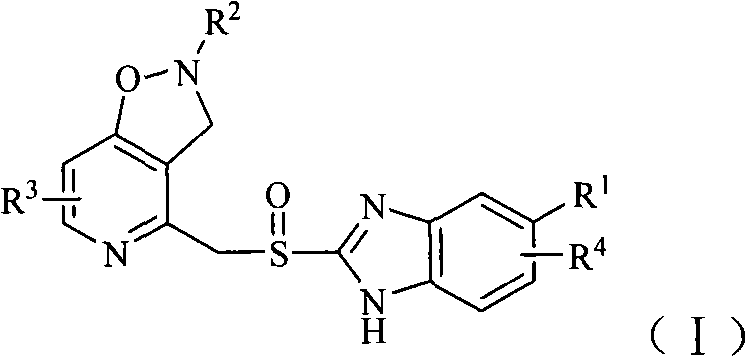
Benzimidazole derivative containing isoxazole-pyridine
A compound and isomer technology, applied in the application field of benzimidazole derivatives and drugs, can solve the problems of affecting drug efficacy and pharmacokinetic parameters, insufficient drug effect, large individual differences in pharmacokinetics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] The preparation of embodiment 1 2-mercapto-benzimidazole
[0105] Put 6.5g (60mmol) of phthalic diamine into the reaction flask, add 200ml of 95% ethanol solution, then add 12.8g (80mmol) of potassium ethoxysulfonate, and heat to reflux at 80°C for 4h. After the reaction was completed, cool to room temperature, pour the reaction solution into 200ml of ice water, stir evenly, adjust the pH to 3-4 with 4N hydrochloric acid, precipitate a solid, filter, wash with water until neutral, and vacuum-dry the filter cake to obtain 7.2g of the product. Rate: 79.7%.
Embodiment 2
[0106] Example 2 Preparation of 2-mercapto-5-methoxy-benzimidazole
[0107] Preparation method Referring to Example 1, 8.3 g (60 mmol) of 4-methoxy o-phenylenediamine and 12.8 g (80 mmol) of potassium ethoxysulfonate were cast. 8.2 g of the product was obtained, yield: 75.4%.
Embodiment 3
[0108] Example 3 Preparation of 2-mercapto-5-difluoromethoxy-benzimidazole
[0109] Preparation method Referring to Example 1, 10.4 g (60 mmol) of 4-difluoromethoxy o-phenylenediamine and 12.8 g (80 mmol) of potassium ethoxysulfonate were injected. 9.5 g of the product was obtained, yield: 73.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com