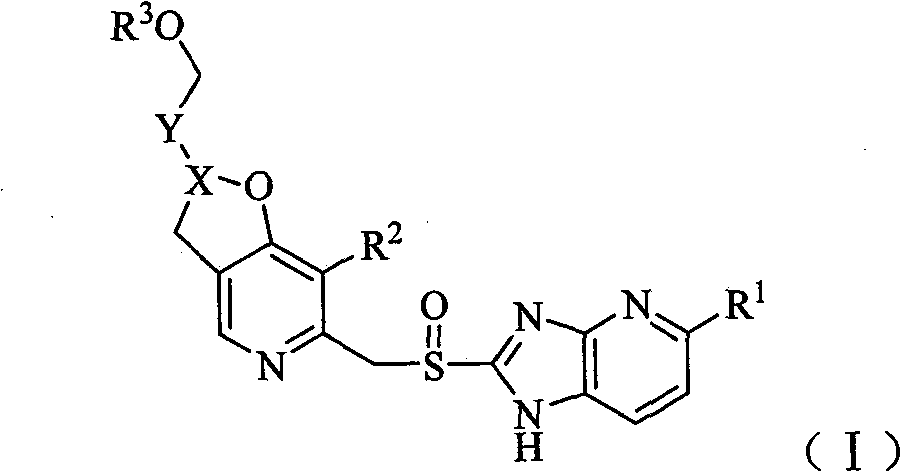
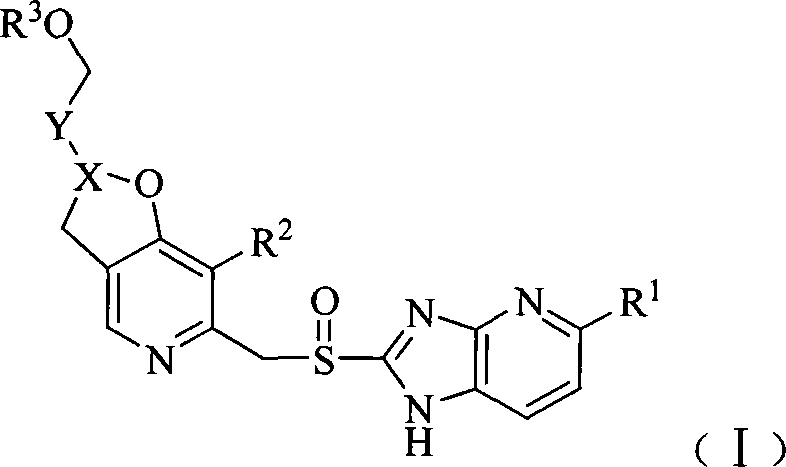
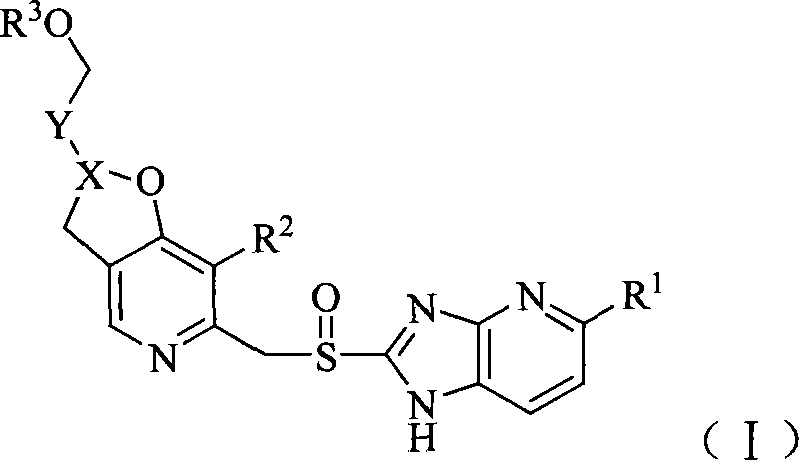
Pyridine methyl sulfinyl imidazopyridine derivative
A methyl and pyridine technology, applied in the field of medicine, can solve the problems of large individual differences in pharmacokinetics, affecting drug efficacy and pharmacokinetic parameters, and slow onset time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1 Preparation of 2-mercapto-5-methoxy-imidazo[4,5-b]pyridine
[0123] 8.3g (60mmol) of 2,3-diamino-6-methoxypyridine was dropped into the reaction flask, 200ml of 95% ethanol solution was added, and 12.8g (80mmol) of potassium ethoxysulfonate was added, and the mixture was heated at 80°C Heat under reflux for 4 hours, after the reaction is complete, cool to room temperature, pour the reaction solution into 200ml of ice water, stir evenly, adjust the pH to 3-4 with 4N hydrochloric acid, precipitate a solid, filter, wash with water until neutral, and vacuum-dry the filter cake to obtain Product 8.5g, yield: 78.2%.
Embodiment 2
[0124] Example 2 Preparation of 1-(4-chloro-5,6-dimethyl-N-oxypyridin-3-yl)-4-methoxy-2-butanol
[0125] Add 300ml chloroform and 73.1g (0.3mol) 1-(4-chloro-5,6-dimethylpyridin-3-yl)-4-methoxy-2-butanol to the reaction flask, cool to 0 ℃, under stirring, add 96g (0.47mol) 85% m-chloroperoxybenzoic acid in batches, continue to stir for 1 hour, then neutralize with saturated solution of sodium bicarbonate, extract with chloroform (150ml×3), extract with Dry over anhydrous sodium sulfate, filter, and distill off the solvent under reduced pressure to obtain 66.4 g of light yellow solid, yield: 85.2%.
Embodiment 3
[0126] Example 3 Preparation of 2-(2-methoxyethyl)-6,7-dimethyl-2,3-dihydrofuro[3,2-c]pyridine oxide
[0127] Under the protection of nitrogen, add 26.0g (100mmol) of 1-(4-chloro-5,6-dimethyl-N-oxide pyridin-3-yl)-4-methoxy- 2-butanol, 100ml of toluene, then add 4g of 60% NaH (mineral oil), slowly raise the temperature to reflux, keep warm and stir for 2h, evaporate the solvent under reduced pressure, add 100ml of chloroform to the residue, and wash with 1N HCl solution, saturated chlorine respectively After washing with sodium chloride solution, 1N sodium hydroxide solution and deionized water, drying over anhydrous sodium sulfate, and concentration to obtain 16.1 g of the product, yield: 72.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com