Multimediator transporter inhibitors for use in treatment of central nervous system disorders
A technology of inhibitors and transporters, applied in nervous system diseases, data processing applications, metabolic diseases, etc., can solve problems such as side effects, intolerance, inability to prevent or cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159] Dosage form matrices can be prepared by methods known in the polymer art. In one method of preparation, 3-5 or more casting compositions are prepared independently, wherein each casting composition contains increasing doses of drug covering each composition from low to high doses. This produces a series of layers that together form a unit polymer matrix with a concentration gradient. In another method of preparation, higher doses are prepared first, followed by layering with layers of decreasing doses, resulting in a polymer matrix with a drug concentration gradient. An example of preparing a dosage form includes mixing a pharmaceutically acceptable carrier such as polyethylene glycol with a known dose of inhibitor, incorporating it into a silicone rubber medical grade elastomer with a crosslinking agent such as stannous octoate, followed by casting in a mold. Repeat this step for subsequent layers.
[0160] The system is allowed to run, for example, for 1 hour, resul...
Embodiment 1
[0314] Example 1: Antagonism and functional activity of dopamine receptors or transporters
[0315] The functional activity of compounds is determined in vitro in cellular assays using recombinant human cell lines. According to the method of Gu et al. (J.Biol.Chem.269:27124, 1994), the functional activity of serotonin uptake inhibition was determined in human HEK-293 cell line, with fluoxetine (EC 50 =57nM) was used as a reference compound. According to the method of Galli et al. (J.Exp.Biol.198:2197,1995), the functional activity of norepinephrine uptake inhibition was determined with MDCK cell line, and desipramine (EC 50 =7nM) was used as a reference compound. To determine dopamine functional activity, the hDAT cell line was used as described by Giros et al. (Mol. Pharmacol. 42:383, 1992), with nomifensine (EC 50 =11 nM) was the reference compound.
[0316] Table 1, human (h) and rat (r) in vitro functional absorption distribution profiles
[0317] compound
...
Embodiment 2
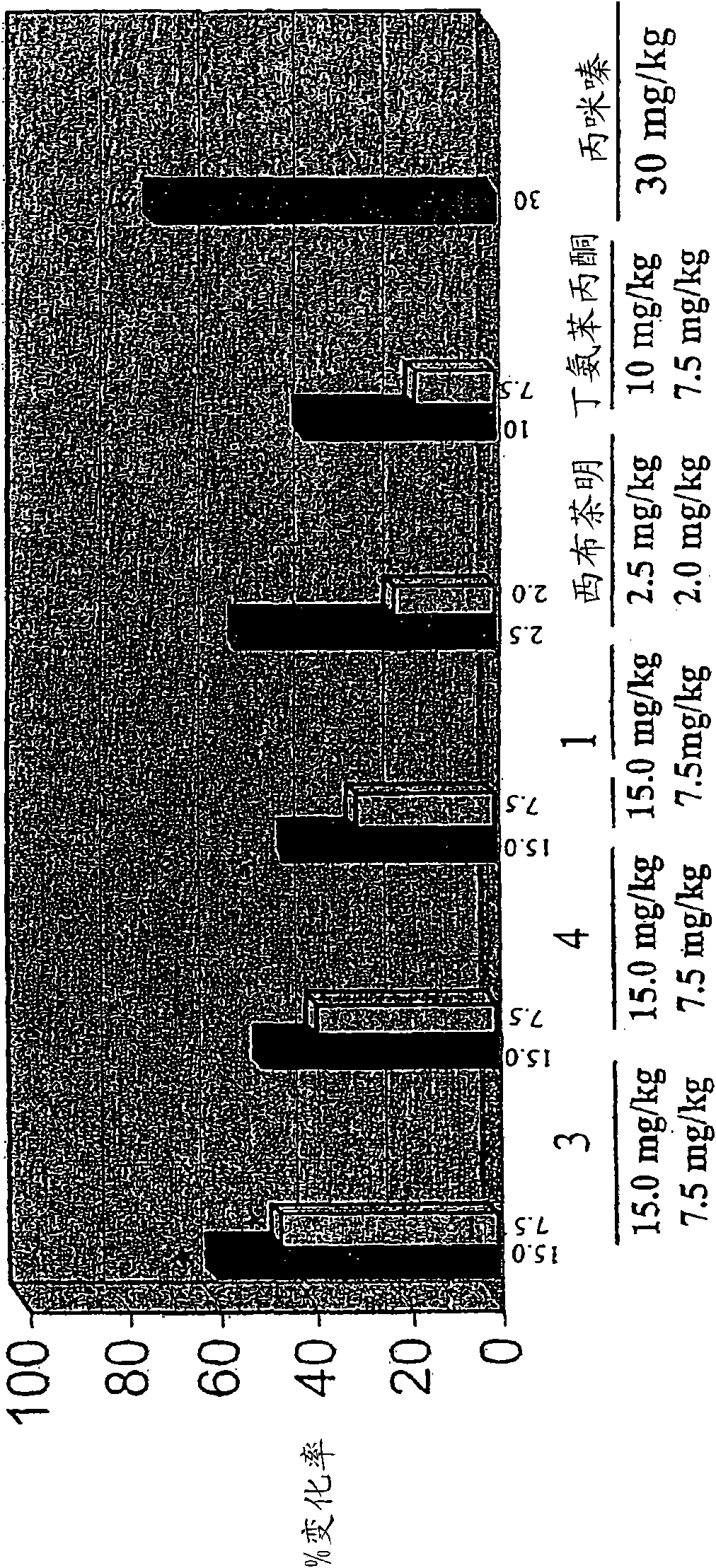
[0335] Example 2: In vivo potency of several exemplary dopamine transporter inhibitors
[0336] The in vivo potency of several exemplary inhibitors (1), (3) and (4) of the invention was determined using a standard forced swim test model in rats. The aim of this study was to evaluate the antidepressant effect of test compounds in a behavioral despair assay in rats using a modified method described in: Porsolt R.D.etal.in Behavioral despair in rats: a new model sensitive to antidepressanttreatment, Eur.J Pharmacol., 47:379-391, 1978; Porsolt et al., Nature 266:730-732, 1977; and Porsolt et al., in Psychopharmacology, Olivier, Mos, and Slangen (eds) Birkhauser Verlag, Basel, pp. .137-159, 1991. In simple terms, when mice (or rats) are forced to swim within a cylinder from which escape is impossible, they readily adopt a characteristic immobility posture and no longer attempt to escape, except for requiring small movements to remain afloat. Immobility has been suggested to refle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com