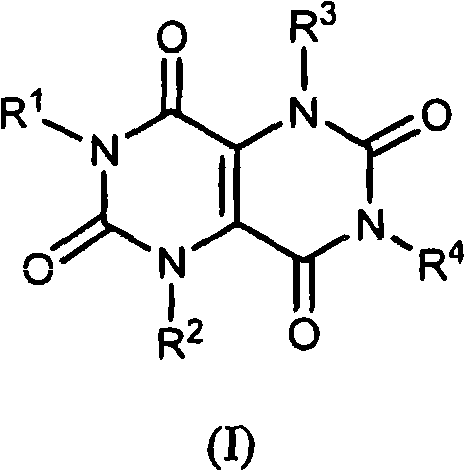
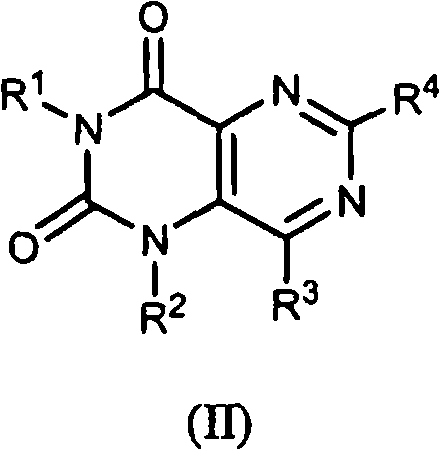
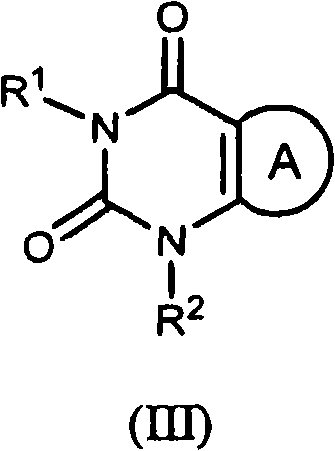
Pyrimidinedione derivatives and methods of use thereof
A technology of compounds and medicinal salts, applied in the direction of drug combinations, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., can solve problems such as diarrhea and skin flushing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0595] Preparation of intermediate compounds 1B and 1C
[0596]
[0597] NaH (1.08 g, 24.70 mmol, 60% in mineral oil) was added to a solution of compound 1A (4.0 g, 23.52 mmol) in DMF / DMSO (30 mL / 20 mL) at room temperature and the resulting mixture was stirred for 40 min . Then n-butyl iodide (2.67 mL, 23.52 mmol) was added and the reaction was stirred at room temperature for about 15 hours. The reaction mixture was then poured into a mixture of EtOAc (250 mL) and 0.5N HCl and the organic phase was washed successively with water (3 times) and brine, then dried (Na 2 SO 4 ), filtered and concentrated in vacuo. The resulting residue was purified using flash column chromatography on silica gel (eluted with hexane / EtOAc (v / v = 1 / 1)) to afford monoalkylated compound 1B (1.4 g, 26%) and dialkylated product 1C ( 1.7 g, 26%). Electrospray MS[M+1] + 240.1.
Embodiment 2
[0599] Preparation of intermediate compound 2B
[0600]
[0601] Step A - Synthesis of Compound 2A
[0602]
[0603] NaH (92.9 mg, 2.12 mmol, 60% in mineral oil) was added to a solution of compound IB (0.4 g, 1.77 mmol) in DMF (3.5 mL) at room temperature and the resulting mixture was stirred for 40 min. Then iodomethane (0.165 mL, 2.65 mmol) was added and the reaction mixture was stirred at room temperature overnight, then poured into a mixture of EtOAc (100 mL) and 0.5N HCl. The organic phase was washed successively with water (3 times) and brine, then dried (Na 2 SO 4 ), filtered and concentrated in vacuo. The resulting residue was purified using silica gel flash column chromatography (eluting with hexane / EtOAc (v / v = 2 / 1 )) to provide compound 2A (0.39 g, 92%).
[0604] Step B - Synthesis of Compound 2B
[0605]
[0606] HNO 3 (0.6mL, fuming) was added dropwise to compound 2A (0.39g, 1.62mmol) in H 2 SO 4 (2.4 mL, 96%) in solution at 0°C. The mixture was ...
Embodiment 3
[0608] Preparation of intermediate compound 3A
[0609]
[0610] Compound 3A was prepared using the method described in Example 2, Step B, substituting Compound 1B for Compound 2A. Electrospray MS[M+1] + 271.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com