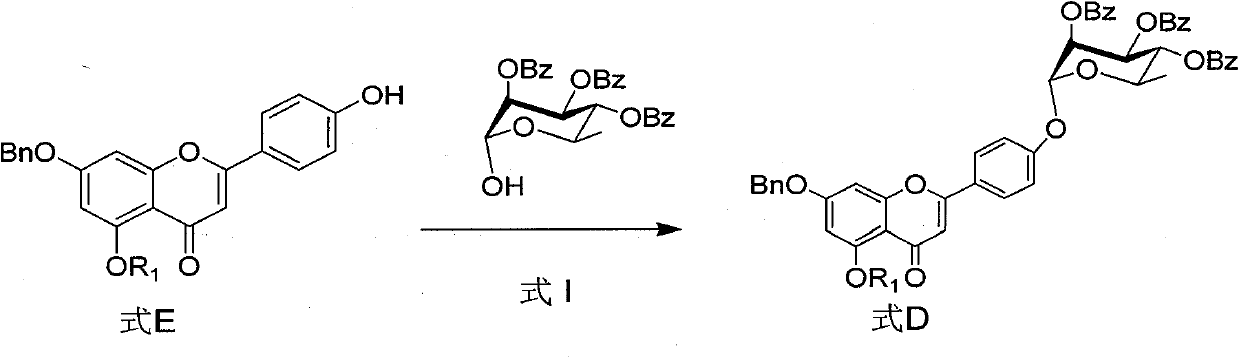
Intermediate of flavonoid compound and preparation method and application thereof
A technology of flavonoids and compounds, applied in the direction of sugar derivatives, organic chemistry, drug combination, etc., can solve the problems of low extraction efficiency, complicated and cumbersome operation of separating and extracting compounds of formula A, and difficulty in satisfying research applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Preparation of formula M compound:
[0058] Reference example 1 5,7, the synthesis of 4 '-O-trihexanoyl apigenin (formula M compound, R 1 for -CO(CH 2 ) 4 CH 3 )
[0059] Apigenin (Formula G, R 1 for -CO(CH 2 ) 4 CH 3 , 1.08g, 4mmol), 4-dimethylaminopyridine (155mg, 1.2mmol), triethylamine (2.6ml, 20mmol,) were dissolved in 10ml of dimethylformamide, hexanoyl chloride (3.2ml, 22.8mmol), stirring reaction under 25 ℃ of temperature 8 hours (TLC detects that apigenin is consumed), system is diluted with dichloromethane, organic phase is washed twice with saturated brine, dried over anhydrous sodium sulfate, filters and collects organic phase, After concentration, the crude product was recrystallized from ethanol to obtain a white solid (1.95 g, yield 88%).
[0060] 1 H NMR (400MHz, CDCl3) δ: 0.91(m, 9H); 1.34(m, 12H); 1.75(m, 6H); 2.57(m, 4H); 2.76(d, 2H); 6.62(s, 1H) ;6.83(s,1H);7.26(d,2H);7.33(s,1H);7.88(d,2H)
[0061] MS: 565(M+H); 1151(2M+Na)
[0062] Refe...
Embodiment 1
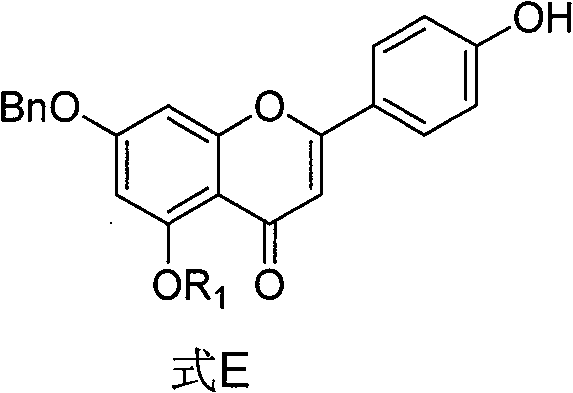
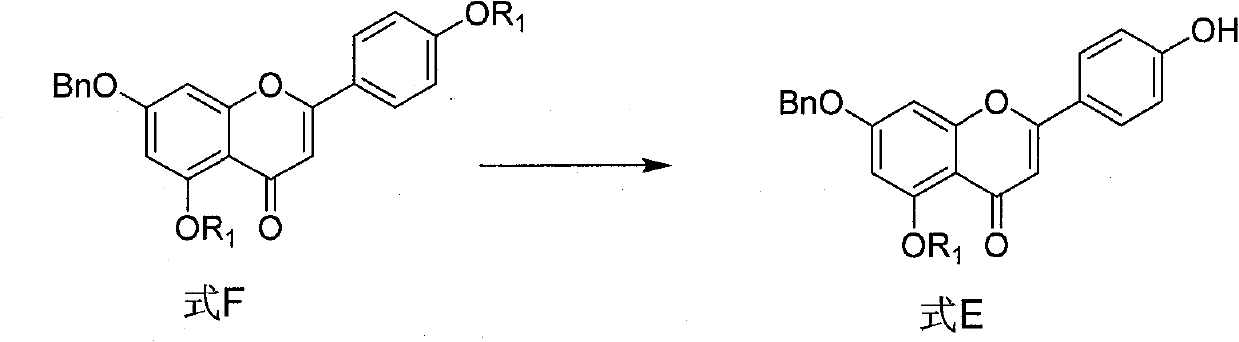
[0080] Embodiment 1 The synthesis (formula E compound, R 1 =-CO(CH 2 ) 4 CH 3 )
[0081] Formula F (n=4, 2g, 3.5mmol) was dissolved in a mixed solvent of 15ml of dichloromethane and 15ml of methanol, after cooling to 0°C, potassium carbonate (69mg, 0.5mmol) was added, and the temperature was naturally raised to 20°C, and reacted for 8 hours (TLC shows that formula F is consumed), add 1mol / L hydrochloric acid methanol to neutralize, remove the solvent by rotary evaporation, separate and purify by silica gel column chromatography (dichloromethane: acetone=30:1) to obtain a light yellow solid (1.52g, 90.3 %).
[0082] 1 H NMR (400MHz, CDCl 3 )δ:0.92(m,3H);1.39(m,4H);1.46(m,2H);2.67(t,2H);5.31(s,2H);6.49(s,1H);6.73(d,1H ); 7.06(d, 2H); 7.21(d, 1H); 7.36(m, 5H); 7.88(d, 2H), 9.02(s, 1H)
[0083] 13 C NMR (CDCl 3 )δ: 13.9, 22.3, 24.1, 31.3, 34.3, 99.9, 105.9, 108.9, 111.0, 116.1, 116.2, 122.2, 127.4, 127.5, 127.8, 128.7, 128.8, 135.3, 150.3, 158.6, 160.1, 162 , 177.0
[...
Embodiment 2
[0085] Example 2 Synthesis of 5-O-hexanoyl-7-O-benzyl-4'-hydroxypigenin (formula E compound, 1 =-CO(CH 2 ) 4 CH 3 )
[0086] Formula F (n=4, 2g, 3.5mmol) was dissolved in a mixed solvent of 7.5ml of dichloromethane and 7.5ml of methanol, cooled to -10°C, added sodium carbonate (53mg, 0.5mmol), and naturally rose to 30°C, Reacted for 10 hours (TLC showed that Formula F was consumed), added 1 mol / L methanol hydrochloric acid to neutralize, removed the solvent by rotary evaporation, and purified by silica gel column chromatography (dichloromethane:acetone=30:1) to obtain a light yellow solid (1.40 g, 83.2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com