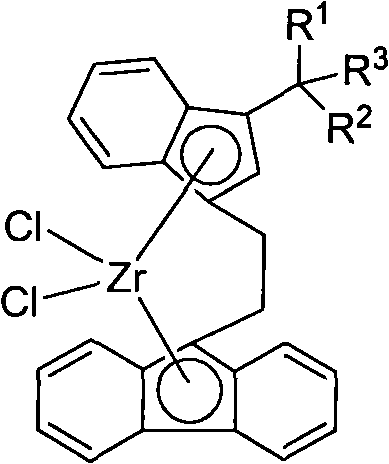
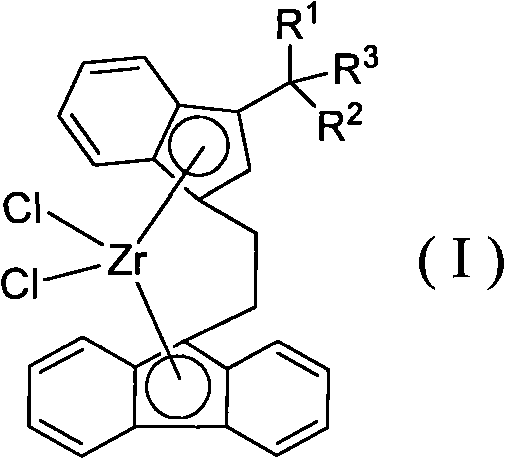
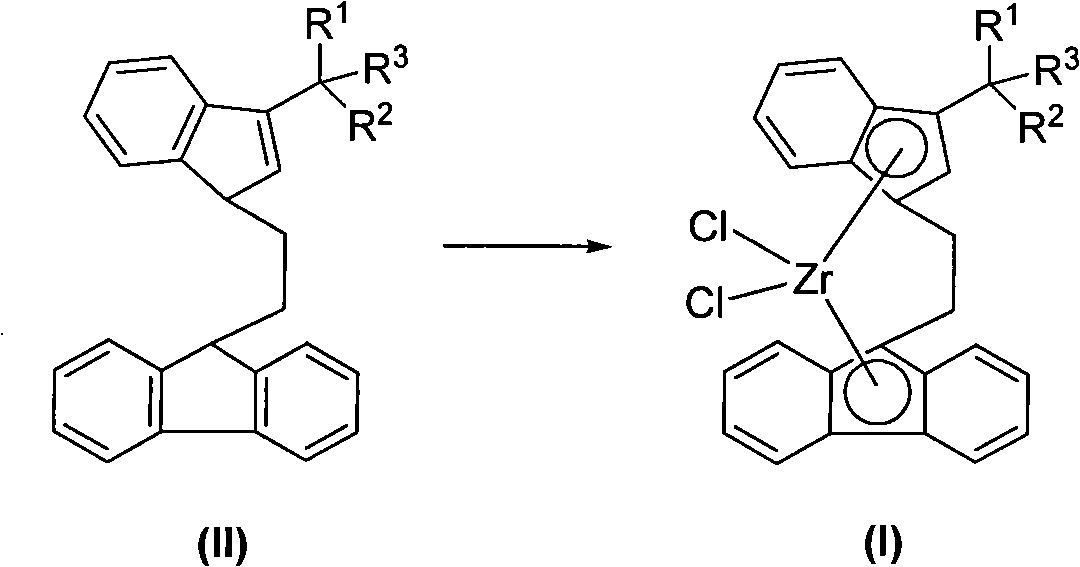
Novel ethidene bridged linkage substituted indene fluorene zirconium compound, method for preparing same and application thereof
A zirconium compound, bisindenyl fluorene technology, applied in the field of preparation and its application in olefin homopolymerization, can solve the problem that the dimerization selectivity of metallocene complexes is not very good, and bridged metallocene complexes have not yet been seen and other problems, to achieve the effect of wide application prospects, stable properties and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of Ligand Compound L1
[0036]
[0037] Add 3.46g of 3-(1-methyl-1-phenyl-ethyl)indene into the reaction flask, add 30mL of petroleum ether, add dropwise 7.0mL (2.15mol / L) of n-butyl lithium solution, dropwise, After stirring for 2 hours, an off-white powdery solid was obtained, which was dissolved by adding 30 mL of diethyl ether. Add dropwise a mixed solution of 3.46g 9-(2-bromo-ethyl)-fluorene and 20mL diethyl ether, stir for 4h, then add 40mL saturated ammonium chloride aqueous solution to stop the reaction, then extract with 100mL diethyl ether, the obtained yellow liquid is washed with anhydrous magnesium sulfate dry. After filtration and recrystallization, 14.40 g of yellow solid L was obtained, with a yield of 81.6%.
[0038] 1 H NMR (400MHz, 298K, CDCl 3 ): δ7.72(d, J=7.6Hz, 2H, Flu-H), 7.48(d, J=7.6Hz, 1H, Ar-H), 7.41(d, J=7.6Hz, 1H, Ar-H ), 7.33(t, J=7.6Hz, 2H, Ar-H), 7.30-7.26(m, 4H, Ar-H), 7.23-7.18(m, 3H, Ar-H), 7.13(d, J= 6.8Hz, 1H, Ar-...
Embodiment 2
[0040] Synthetic Ligand Compound L2
[0041]
[0042] Add 3.25g of 3-[1-methyl-1-(2-methyl-phenyl)-ethyl]indene into the reaction flask, add 30mL of petroleum ether, and dropwise add 7.0mL (2.15mol / L) of n-butyl Lithium solution, dropwise, stirred for 2h, and the powdery solid was dissolved with 30mL ether. Add dropwise a mixed solution of 3.00g 9-(2-bromo-ethyl)-fluorene and 20mL diethyl ether, stir for 4h, then add 40mL saturated ammonium chloride aqueous solution to stop the reaction, then extract with 100mL diethyl ether, and the obtained yellow liquid is washed with anhydrous magnesium sulfate dry. After filtration and recrystallization, 23.04 g of a yellow solid was obtained, with a yield of 62.8%.
[0043] 1 H NMR (400MHz, 298K, CDCl 3 ): δ7.77 (dd, J=7.6, 3.6Hz, 2H, Flu-H), 7.60 (d, J=7.6Hz, 1H, Ar-H), 7.54 (d, J=7.2Hz, 1H, Ar -H), 7.45(d, J=7.2Hz, 1H, Ar-H), 7.41-7.32(m, 5H, Ar-H), 7.21(d, J=7.2Hz, 1H, Ar-H), 7.15 (t, J=7.2Hz, 1H, Ar-H), 7.04(t, J=7.4Hz, 1H, ...
Embodiment 3
[0045] Synthetic Ligand Compound L3
[0046]
[0047] Add 1.70g of 3-[1-methyl-1-(2-methoxy-phenyl)-ethyl]indene to the reaction flask, add 30mL petroleum ether, drop 3.5mL (2.15mol / L) n-butyl Lithium solution, dropwise, stirred for 2h, filtered, and the powdery solid was dissolved in 30mL ether. Add dropwise a mixed solution of 1.75g 9-(2-bromo-ethyl)-fluorene and 20mL diethyl ether, stir for 4h, then add 40mL saturated ammonium chloride aqueous solution to stop the reaction, then extract with 100mL diethyl ether, and the obtained yellow liquid is washed with anhydrous magnesium sulfate dry. After filtration and recrystallization, 1.24 g of yellow solid L was obtained, with a yield of 42.1%.
[0048] 1 H NMR (400MHz, 298K, CDCl 3 ): δ7.78 (d, J=7.2Hz, 2H, Flu-H), 7.54 (d, J=7.6Hz, 1H, Ar-H), 7.49 (d, J=7.4Hz, 1H, Ar-H ), 7.43-7.37(m, 3H, Ar-H), 7.33(dt, J=7.4, 1.2Hz, 2H, Ar-H), 7.24-7.20(m, 2H, Ar-H), 7.03(dt, J=7.4, 0.9Hz, 1H, Ar-H), 6.98-6.93(m, 2H, Ar-H), 6.76(d...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap