Substituted piperazines as cb1 antagonists
A kind of piperazine, C3-C10 technology, applied in the field of substituted piperazine as CB1 antagonist
Inactive Publication Date: 2010-07-28
INTERVET INT BV
View PDF111 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, none of the disclosed compounds have substituted aryl and / or heteroaryl substituents at both the 1 and 2 positions of the piperazine ring
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
[0974] The compounds of formula (I) shown in the table below were prepared following one or more of the methods reported above.
[0975]
[0976]
[0977]
[0978]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
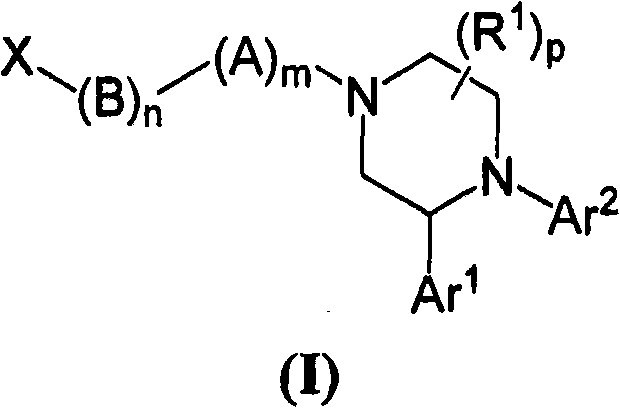
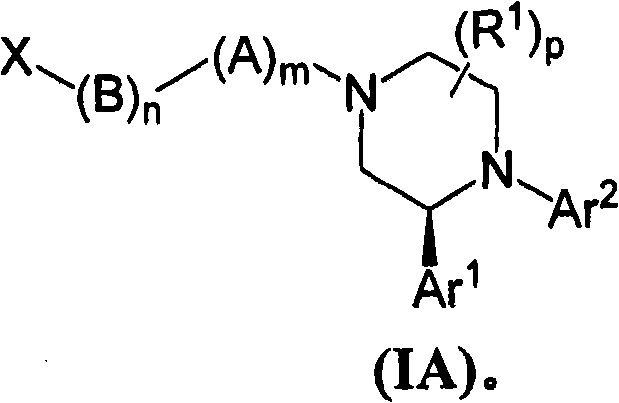
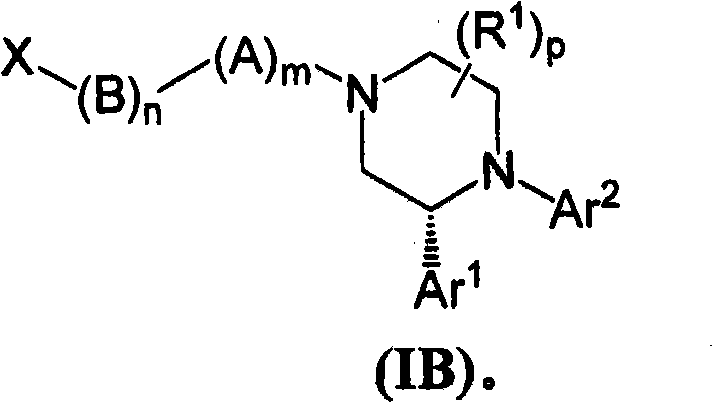
Compounds of Formula (I): or pharmaceutically acceptable salts, solvates, or esters thereof, are useful in treating diseases or conditions mediated by CB1 receptors, such as metabolic syndrome and obesity, neuroinflammatory disorders, cognitive disorders and psychosis, addiction (e.g., smoking cessation), gastrointestinal disorders, and cardiovascular conditions.
Description
[0001] prior application [0002] This application claims the benefit of priority to Application No. 60 / 946,896, filed June 28, 2007, which is incorporated by reference in its entirety. Background of the invention [0003] CB 1 The receptor is one of the most abundant neuromodulatory receptors in the brain and is expressed at high levels in the hippocampus, cortex, cerebellum, and basal ganglia (eg, Wilson et al., Science, 2002, Vol. 296, 678-682). Selective CB 1 Receptor antagonists, e.g. pyrazole derivatives such as rimonabant (e.g. US6,432,984) are useful in the treatment of various conditions such as obesity and metabolic syndrome (e.g. Bensaid et al., Molecular Pharmacology, 2003 Vol. 63, No. 4, pp. 908-914; Trillou et al., Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002 Vol. 284, R345-R353; Kirkham, Am. J. Physiol. Regul .Integr.Comp.Physiol.2002 Vol. 284, R343-R344), neuroinflammatory diseases (eg Adam et al., Expert Opin. Ther. Patents, 2002, Vol. 12, No. 10, 14...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D241/04C07D401/06A61K31/495A61P3/00
CPCC07D401/06C07D241/04A61P1/00A61P1/08A61P1/12A61P1/16A61P3/00A61P3/04A61P3/10A61P9/00A61P9/10A61P13/02A61P15/08A61P25/06A61P25/08A61P25/14A61P25/16A61P25/18A61P25/22A61P25/24A61P25/28A61P25/32A61P25/34A61P25/36A61P29/00A61P31/00A61P35/00A61P43/00
Inventor E·J·吉尔伯特W·J·格林李S·W·李M·W·米勒J·D·斯科特A·斯坦福德C·S·西利
Owner INTERVET INT BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com