C genotype entecavir-resistant mutation HBV stable replication and expression cell line
A cell line and C gene technology, applied in genetic engineering, plant genetic improvement, cells modified by introducing foreign genetic material, etc., can solve the problem of affecting virus phenotype, not fully reflecting the phenotypic characteristics of drug-resistant strains, and drug resistance rate Increase and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
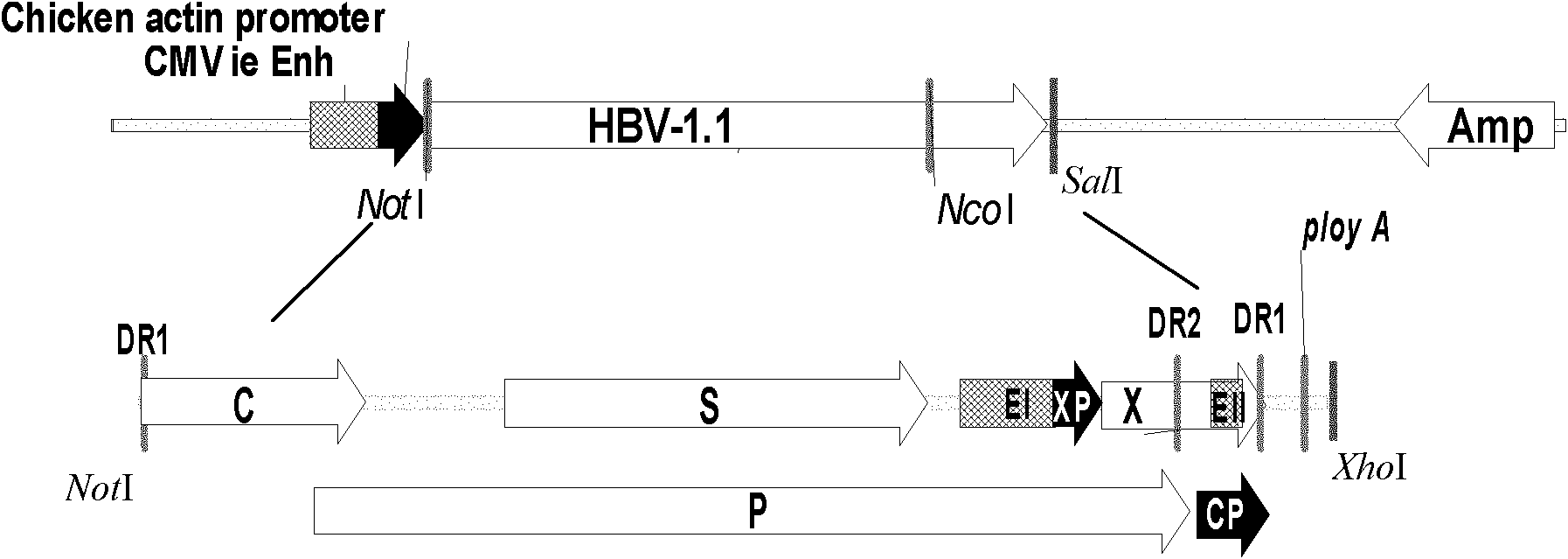
[0040] Example 1 Construction of 1.1 times longer HBV expression vector
[0041] (1) Preparation of full-length circular HBV template The full-length HBV genome (S1145) cloned in the pGEM-Teasy vector was digested with BspQI / ScaI, purified and recovered a 3.2kb fragment, and 10 μL of the digested product was ligated with T4 DNA Enzyme ligation was performed overnight, and the ligation product was used for later use.
[0042] (2) Amplification of double HBV fragments Referring to the S1145 sequencing results, two pairs of primers for amplifying HBV were designed and synthesized according to the literature [Jiang Linbin, et al. PLA Medical Journal, 2010, 35:635-638] (Table 1). Take 5 μl of the circularized HBV DNA product as a PCR template, use the A / B primer pair to amplify a 2735bp HBV partial genome fragment, and use the C / D primer pair to amplify a 643bp HBV partial genome fragment, respectively clone the AB and CD fragments in pGEM-Teasy vector.
[0043] Table 1 The seque...
Embodiment 2
[0046] Example 2 Stable cell line screening
[0047] (1) Plasmid DNA transfection and cell clone screening Extract the transfection-grade recombinant plasmid pTriEx-1.1-HBV, and quantify the concentration with an ultraviolet spectrophotometer. HepG2 cells were cultured at 37°C in complete DMEM medium containing 5% CO2 and 10% FBS. Before transfection, the cells were inoculated into 24-well plates and cultured overnight. When the cell fusion reached 80%, the DMEM without serum and antibiotics was replaced, and the DNA was introduced with FuGENE HD liposomes. The specific method was carried out according to the operation manual. 6h after transfection, add FBS to 10%. Set untransfected wells as negative controls. Add G418 (final concentration 600 μg / ml) to the cell culture dish for 48 hours for screening, change the medium every 2 days, and replace it with G418 (300 μg / ml) at a maintenance concentration after the cells in the control group are completely dead to continue screen...
Embodiment 3
[0049] Example 3 Detection of the constructed cell lines of the present invention:
[0050] 1. Expression of major HBV antigens
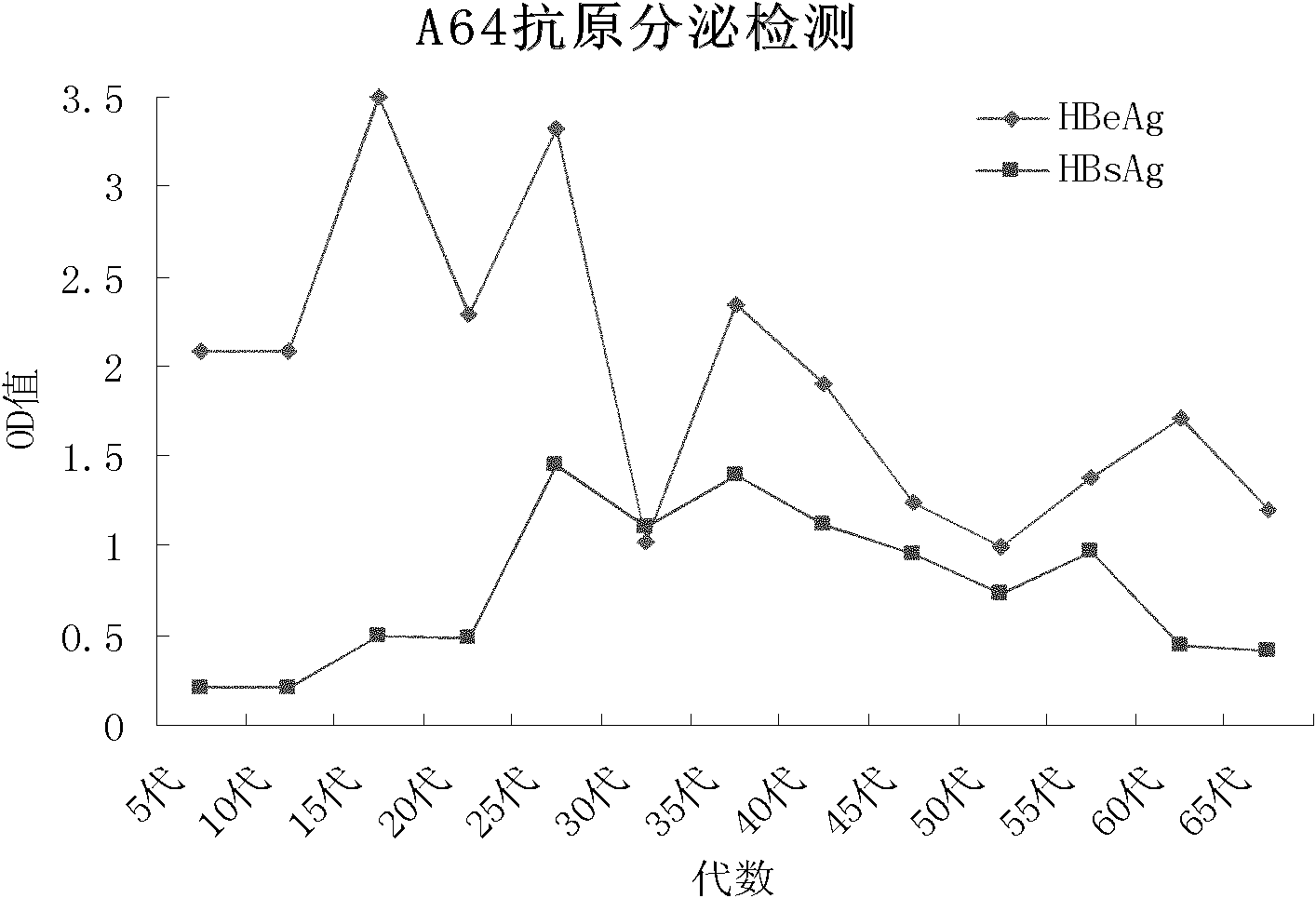
[0051] (1) ELISA detection Collect HepG2.A64 cell line 5th, 10th, 15th, 20th, 25th, 30th, 35th, 40th, 45th, 50th, 55th, 60th generation cell culture supernatant, ELISA (double antibody sandwich method) detection For the expression of HBsAg and HBeAg, the wavelength was set at 450 nm. With the increase of cell passage number, the expression of antigen gradually increased and tended to be stable, the average OD value of HBsAg: 0.67±0.42, HBeAg: 2.03±0.61( figure 2 ).
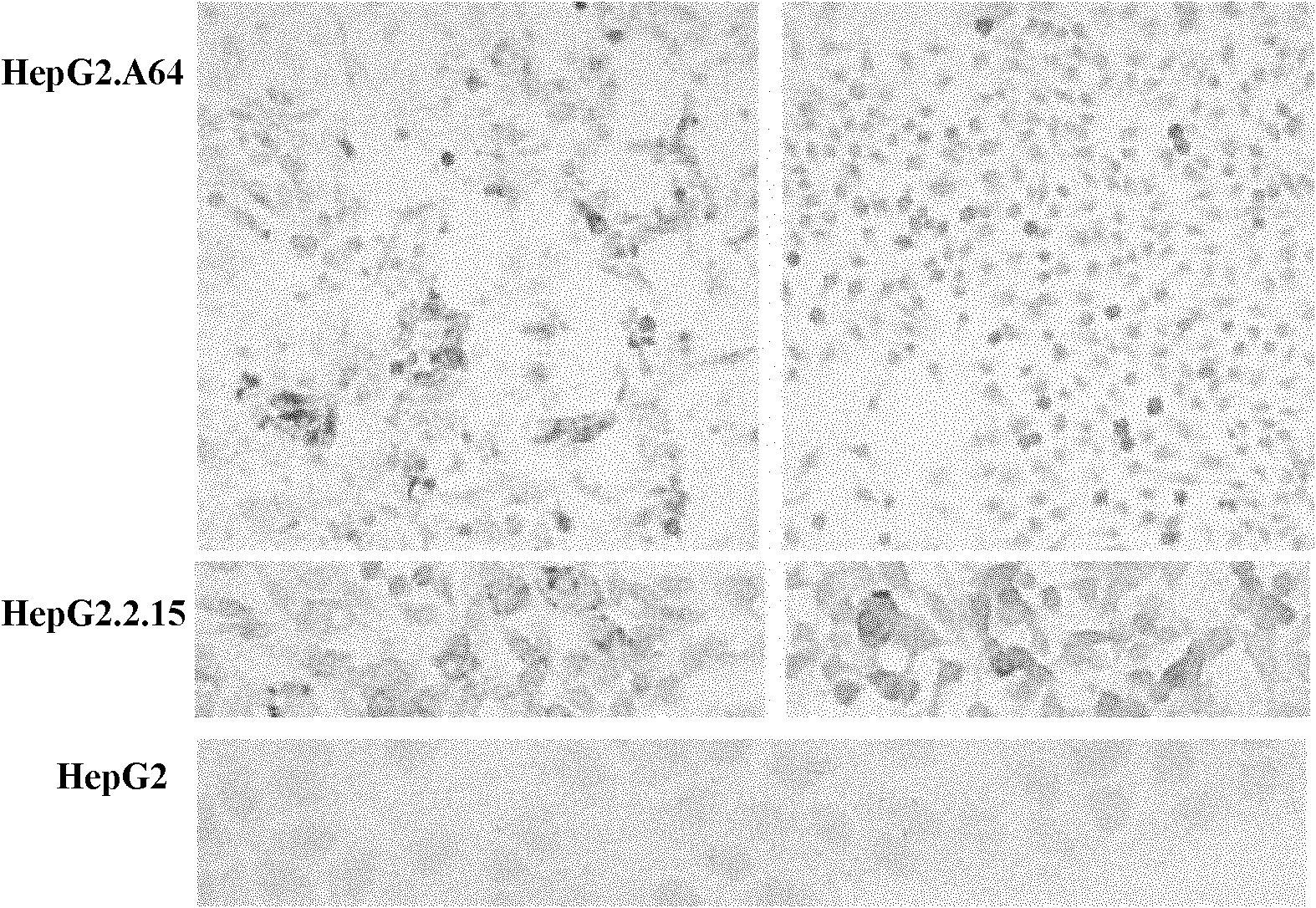
[0052] (2) Immunohistochemistry Put polylysine-treated slides in a 6-well plate, add HepG2.A64 cells 5×10 5 / well, each 2 wells allowed the cells to slide for 72 hours, and after the cells were fixed, the two-step method was used for immunohistochemical staining. The primary antibody was rabbit anti-HBs / anti-HBc, diluted at 1:100; To amplify the binding signal, the secondary antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com