Methods for purifying antibodies using protein a affinity chromatography
A protein and affinity technology, applied in peptide preparation methods, chemical instruments and methods, immunoglobulin, etc., can solve the problems of difficult, unsuccessful and time-consuming purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0081] The present invention provides a first method for purifying monomeric monoclonal antibodies from a sample comprising monomeric monoclonal antibodies, host cell impurities, dimers and higher order aggregates, the method comprising: ( a) exposing the sample to a protein A affinity column; (b) eluting the monomeric monoclonal antibody from the protein A affinity column using elution buffer; and (c) collecting the monomeric monoclonal antibody from step (b). Cloning one or more fractions of the antibody to form a protein A product pool, wherein the product pool (i) comprises less than 5% higher order aggregates, and (ii) has a pH of about 3.5 to about 4.5, whereby Purification of monomeric monoclonal antibodies from samples.
[0082] In one embodiment of the above method, the elution buffer is acetate or citrate.
[0083] In another embodiment, the concentration of citrate in the elution buffer is from about 0.030M to about 0.085M. As used herein, "about" means ± 0.015M. ...
Embodiment 1
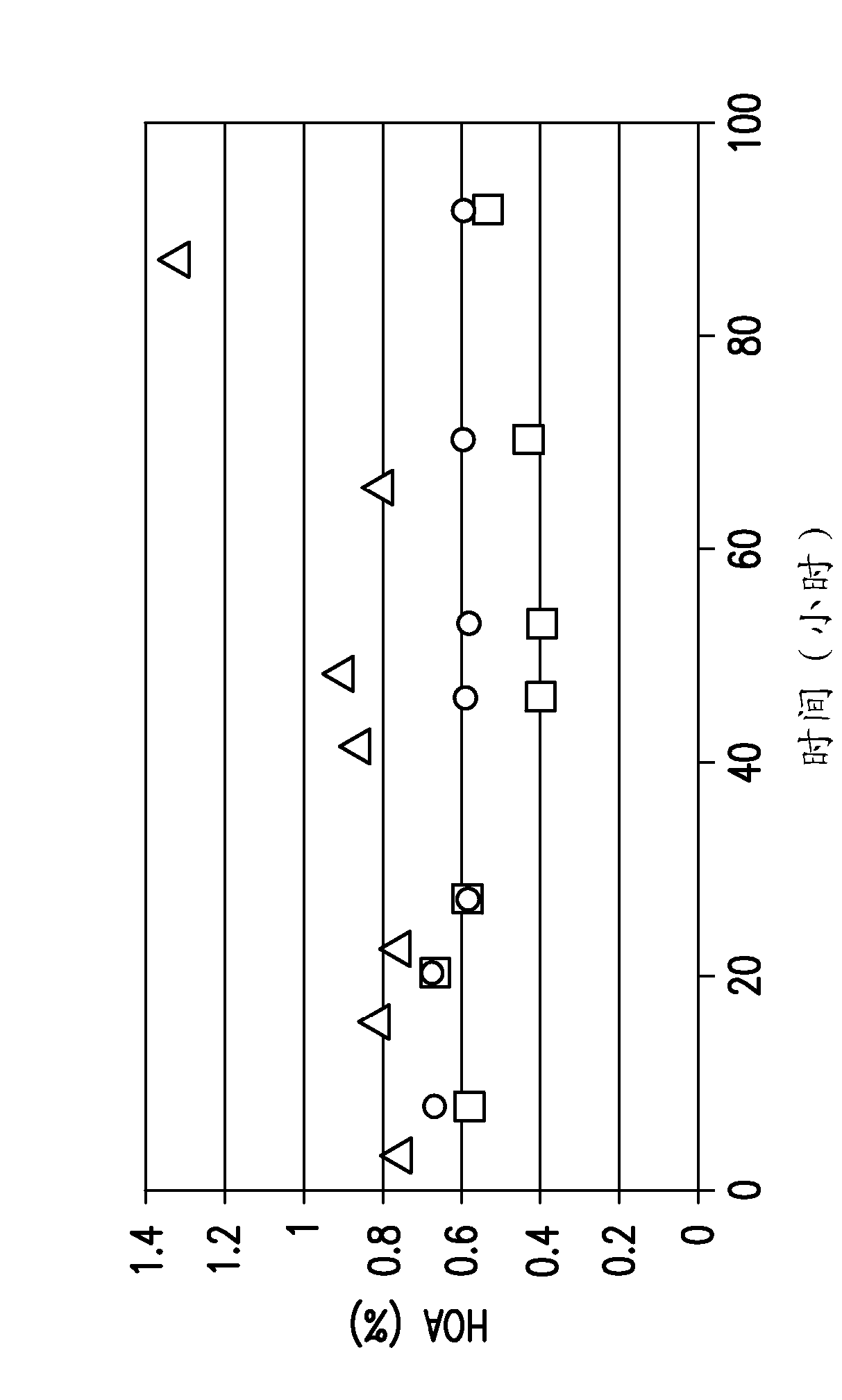
[0130] Reduce temperature to reduce protein aggregate levels during protein A affinity chromatography elution and subsequent low pH hold
[0131] To characterize the temperature and pH dependence of protein aggregation, the effect of temperature and pH on the eluted pool of PAP containing citrate (100mM, pH 3.5) was evaluated against an anti-DKK-1 monoclonal antibody. The same procedure can be used for other mAbs, such as anti-ADDL monoclonal antibodies.
[0132] Materials and methods
[0133] Small scale: with AKTA EXPLORER 100 TM , for all small-scale experiments. Phosphate, citrate, and sodium hydroxide buffers were purchased from Hyclone (Logan, UT). Tris base for pH adjustment of PAP was purchased from Hyclone (Logan, UT). Mabselect for Protein A Affinity Chromatography Experiments TM Resins were purchased from GE Healthcare. A depth-filtered centrate was obtained and used as starting material for protein A affinity chromatography experiments. A Thermomixer R (...
Embodiment 2
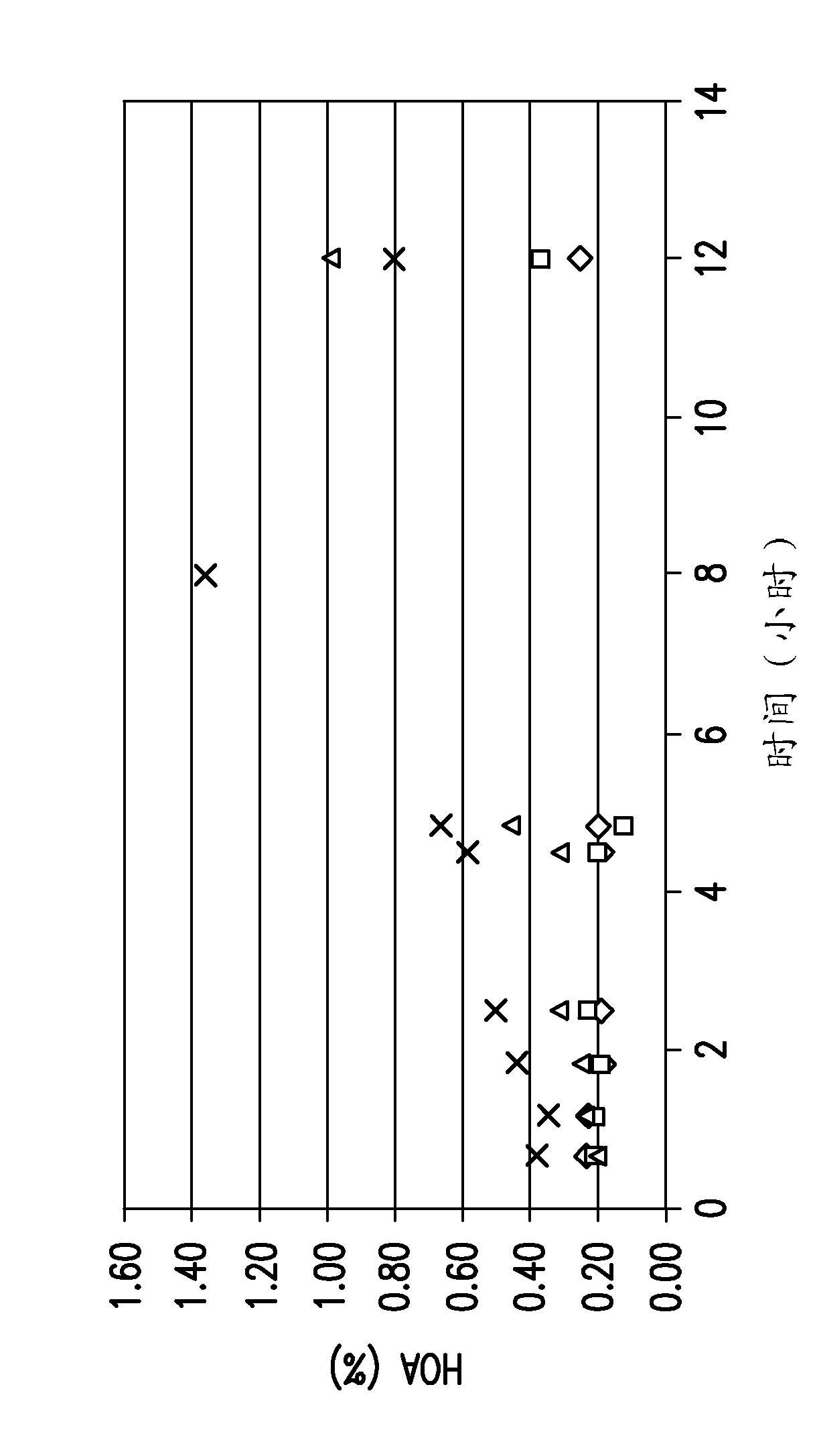
[0145] Raising the pH of the PAP pool to reduce protein aggregate levels during protein A affinity chromatography elution and subsequent low pH hold
[0146] To characterize the effect of pH on protein aggregation, the effect of pH (3.5-5.0) on the QPAP eluted pool was evaluated against an anti-DKK1 monoclonal antibody. The same procedure can be used for other mAbs, such as anti-ADDL monoclonal antibodies.
[0147] Materials and methods
[0148] Small scale: with AKTA EXPLORER 100 TM (GE Healthcare), for all small-scale experiments. Phosphate, citrate, and sodium hydroxide buffers were purchased from Hyclone (Logan, UT). Tris base for pH adjustment of PAP was purchased from Hyclone (Logan, UT). Mabselect for Protein A Affinity Chromatography Experiments TM Resins were purchased from GE Healthcare. Use depth-filtered centrate as starting material for protein A affinity chromatography experiments. Thermomixer R (Eppendorf) and 2 temperature-controlled chambers were u...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap