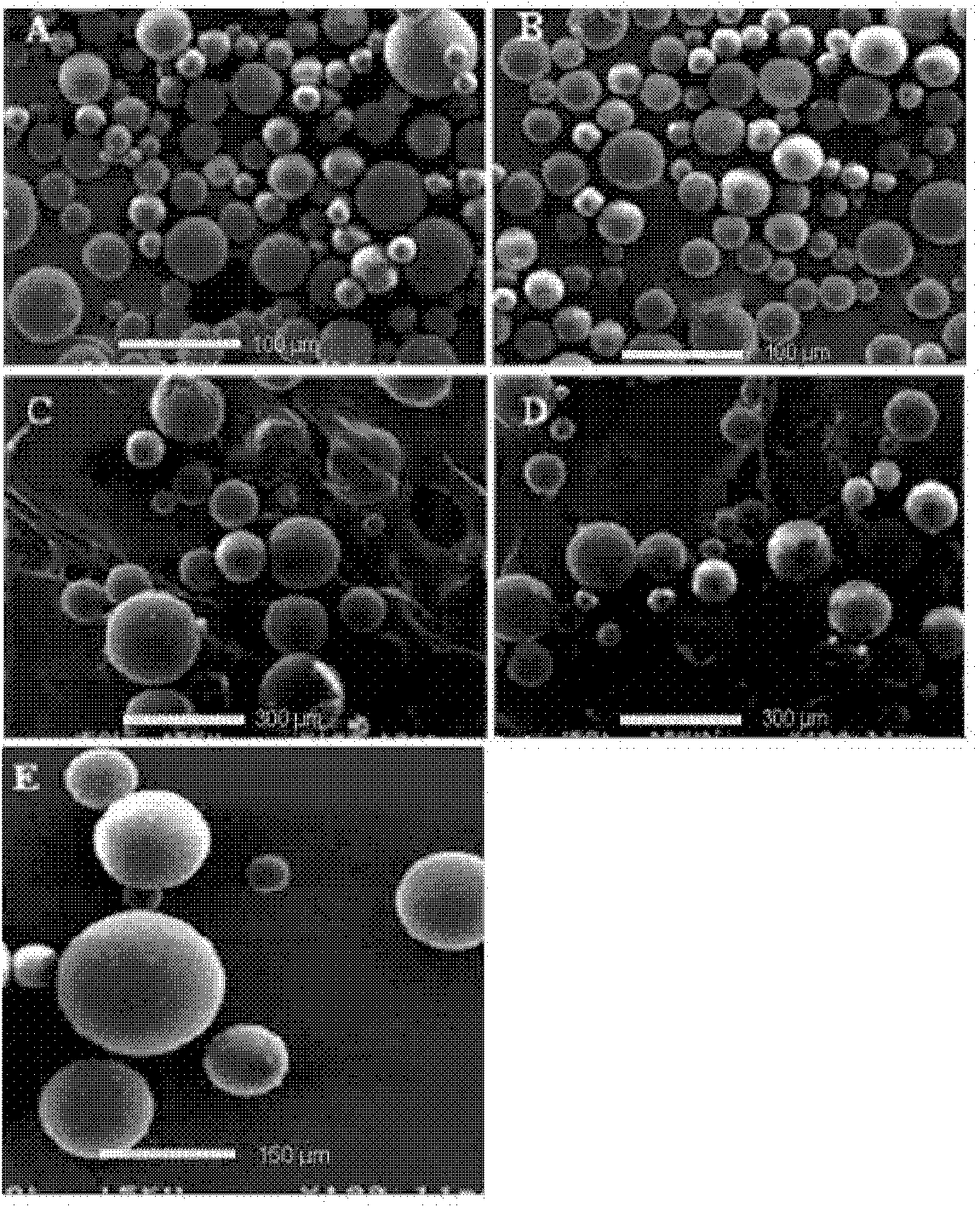
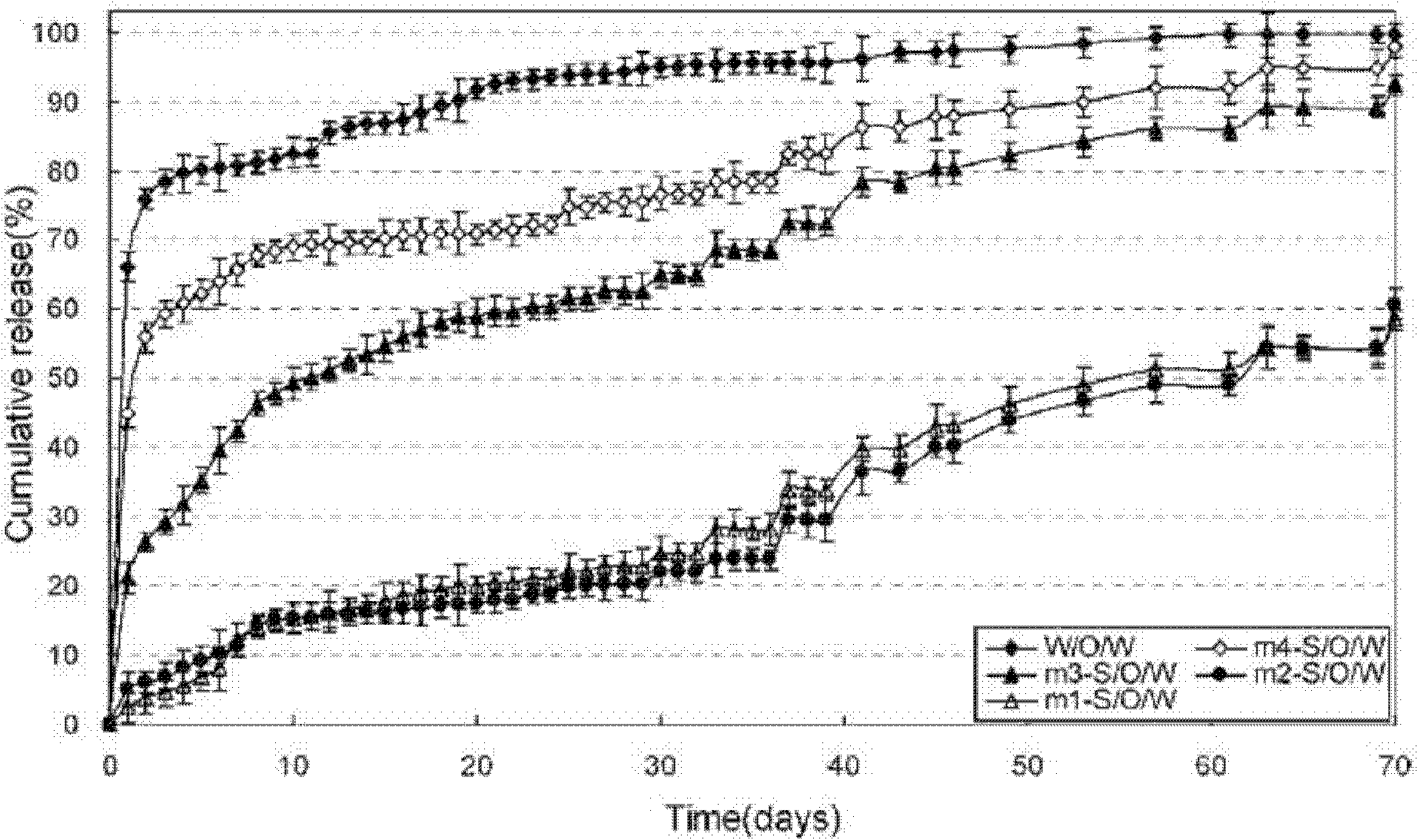
Method for preparing granulocyte-macrophage colony stimulating factor microsphere
A technology of colony-stimulating factor and macrophages, which is applied in pharmaceutical formulas, medical preparations containing active ingredients, peptide/protein components, etc., can solve problems such as inability to overcome the encapsulation rate, achieve regular particles without adhesion, and improve curative effect , the effect of reducing the cost of preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: A method for preparing granulocyte-macrophage colony-stimulating factor (GM-CSF) microspheres by the glycerol method
[0026] (1) Take 10 mg of granulocyte-macrophage colony-stimulating factor dextran particles of 5 μm and a dichloromethane solution of polylactic acid-glycolic acid (50:50, PLGA) with a molecular weight of 47,000 at a concentration of 20% by weight Stir, vortex or sonicate 1mL for 1-5 minutes to form a uniform suspension, that is, solid-in-oil (S / O) emulsion;
[0027] (2) Add the emulsion obtained in step (1) dropwise to the glycerin-modified aqueous phase (W) (W) containing 20% by weight and stir, vortex or sonicate for 0.1-5 minutes to form double emulsion;
[0028] (3) The double emulsion of step (2) is added dropwise in the 1000mL sodium chloride solution that the weight percent concentration is 5% (w / w) and stirs 2-4 hour;
[0029] (4) Collect the microspheres obtained in step (3) by centrifugation, wash with water for 3-5 times, and fre...
Embodiment 2
[0032] Embodiment 2: A kind of method for preparing granulocyte-macrophage colony-stimulating factor (GM-CSF) microspheres by glycerol method
[0033] (1) Take 50 mg of 10 μm granulocyte-macrophage colony-stimulating factor dextran particles and 1 mL of a dichloromethane solution of 20% polylactic acid-glycolic acid (50:50, PLGA) with a molecular weight of 47,000 and stir , Vortex or ultrasonic for 1-5 minutes to form a uniform suspension, that is, solid-in-oil (S / O) emulsion;
[0034] (2) Add the emulsion obtained in step (1) dropwise to the glycerin-modified aqueous phase (W) (W) containing a concentration of 40% by weight and stir, vortex or sonicate for 0.1-5 minutes to form a double emulsion;
[0035] (3) The double emulsion of step (2) is added dropwise in the 1000mL sodium chloride solution that the weight percent concentration is 10% (w / w) and stirs 2-4 hour;
[0036] (4) Collect the microspheres obtained in step (3) by centrifugation, wash with water for 3-5 times, a...
Embodiment 3
[0039] Embodiment three: a kind of method for preparing granulocyte-macrophage colony-stimulating factor (GM-CSF) microspheres by glycerol method
[0040] (1) Take 5 mg of granulocyte-macrophage colony-stimulating factor dextran particles of 5 μm and 1 mL of a dichloromethane solution of polylactic acid (PLA) with a molecular weight of 83,000 at a concentration of 20% and stir, vortex or sonicate for 1-5 A uniform suspension is formed within minutes, that is, a solid-in-oil (S / O) emulsion;
[0041] (2) Add the emulsion obtained in step (1) dropwise to the glycerin-modified aqueous phase (W) (W) containing 60% by weight and stir, vortex or sonicate for 0.1-5 minutes to form double emulsion;
[0042] (3) The double emulsion of step (2) is added dropwise in the 1000mL sodium chloride solution that the weight percent concentration is 1% (w / w) and stirs 2-4 hour;
[0043] (4) Collect the microspheres obtained in step (3) by centrifugation, wash with water for 3-5 times, and freeze...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com