Preparation method of telmisartan intermediate and intermediate compound
A technology of telmisartan and compounds, which is applied in the preparation of telmisartan intermediates and the field of intermediate compounds, can solve the problems of low yield, long reaction steps, cumbersome operation, etc., and achieve high yield, cost reduction, The operation method is simple and convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
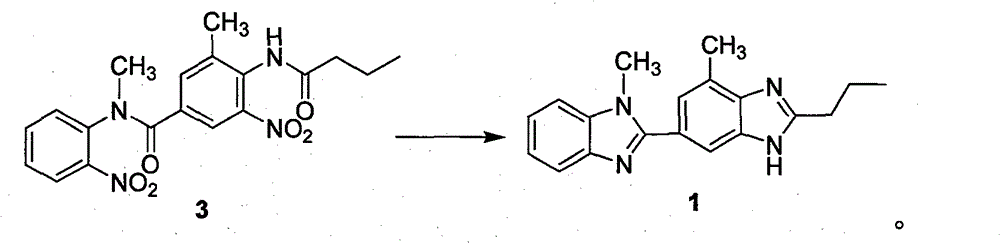
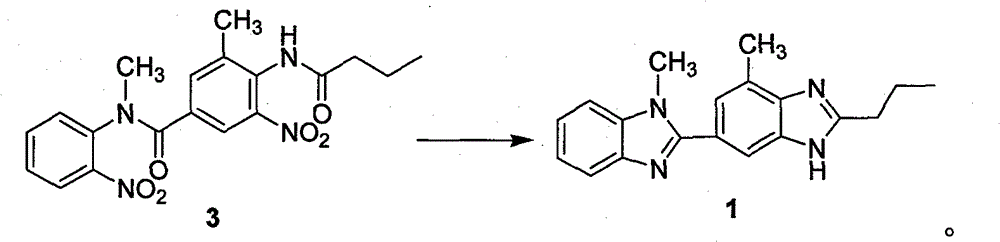
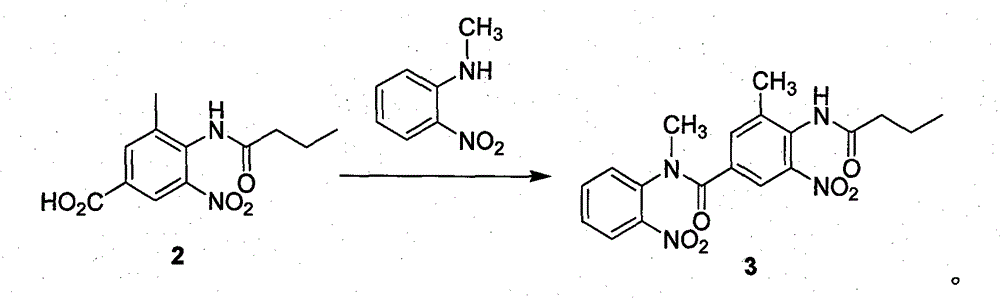
[0033] Example 1 Preparation of N-methyl-N-(2-nitrophenyl)-N-(3-methyl-4-n-butyramide-5-nitrophenyl) formamide (compound 3)
[0034] 3-Methyl-4-n-butyramide-5-nitrobenzoic acid (21.2g, 0.08mol) was added into 600mL of dichloromethane and 100mL of thionyl chloride, heated and refluxed for 20h, and the intermediate obtained by concentration was dissolved directly without separation. In 100mL of DMF, add o-nitrobenzylamine (6.1g, 0.04mmol) into the reaction flask, stir at room temperature for 2d, add 500mL of dichloromethane, add dropwise 200mL of 2N sodium hydroxide solution, stir at room temperature for 1h, and separate , the aqueous layer was extracted with 200 mL of dichloromethane, the combined organic layers were washed with saturated brine, dried, filtered and concentrated to obtain a crude product that was recrystallized with ethyl acetate and petroleum ether to obtain 13.3 g of a light yellow powder with a yield of 83.1%.
[0035] H NMR (300MHz, CDCl 3 )δ: 8.23 (br, 1...
Embodiment 2
[0037] Example 2 Preparation of N-methyl-N-(2-nitrophenyl)-N-(3-methyl-4-n-butyramide-5-nitrophenyl) formamide (compound 3)
[0038]3-Methyl-4-n-butyramide-5-nitrobenzoic acid (2.1g, 8mmol) was added to 100mL of dichloromethane and 10mL of thionyl chloride, heated and refluxed for 20h, and the intermediate obtained by concentration was directly dissolved in In 20mL of DMF, add o-nitrobenzylamine (1.2g, 8mmmol) into the reaction flask, stir at room temperature for 2d, add 50mL of dichloromethane, add dropwise 2N sodium hydroxide solution 40mL, stir at room temperature for 1h, separate liquid, water The layers were extracted with 500 mL of dichloromethane, the combined organic layers were washed with saturated brine, dried, filtered and concentrated to obtain a crude product that was separated by column chromatography to obtain 1.4 g of light yellow powder with a yield of 43.6%.
[0039] HPLC: 95.1% NMR data are the same as in Example 1.
Embodiment 3
[0040] Example 3 Preparation of N-methyl-N-(2-nitrophenyl)-N-(3-methyl-4-n-butyramide-5-nitrophenyl) formamide (compound 3)
[0041] Add 3-methyl-4-n-butyramide-5-nitrobenzoic acid (21.2g, 0.08mol) into 600mL of dichloromethane and 60mL of phosphorus trichloride, heat up and reflux for 20h, and concentrate the obtained intermediate directly into the solution without separation. In 100mL of DMF, add o-nitrobenzylamine (6.1g, 0.04mmol) into the reaction flask, stir at room temperature for 2d, add 500mL of dichloromethane, add dropwise 200mL of 2N sodium hydroxide solution, stir at room temperature for 1h, and separate , the aqueous layer was extracted with 200 mL of dichloromethane, the organic layers were combined and washed with saturated brine, dried, filtered and concentrated to obtain a crude product that was recrystallized with toluene and petroleum ether to obtain 10.1 g of a light yellow powder with a yield of 63.1%.
[0042] HPLC: 92.1% NMR data are the same as in Examp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com