New tigecycline crystal form and preparation method thereof
A technology of tigecycline and its crystal form, which is applied in the field of new tigecycline crystal form and its preparation, can solve the problems of limited medical use, inconvenient transportation or storage, unstable tigecycline powder, etc., and achieves good results. Stability, good for transportation and long-term storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of tigecycline crystal form
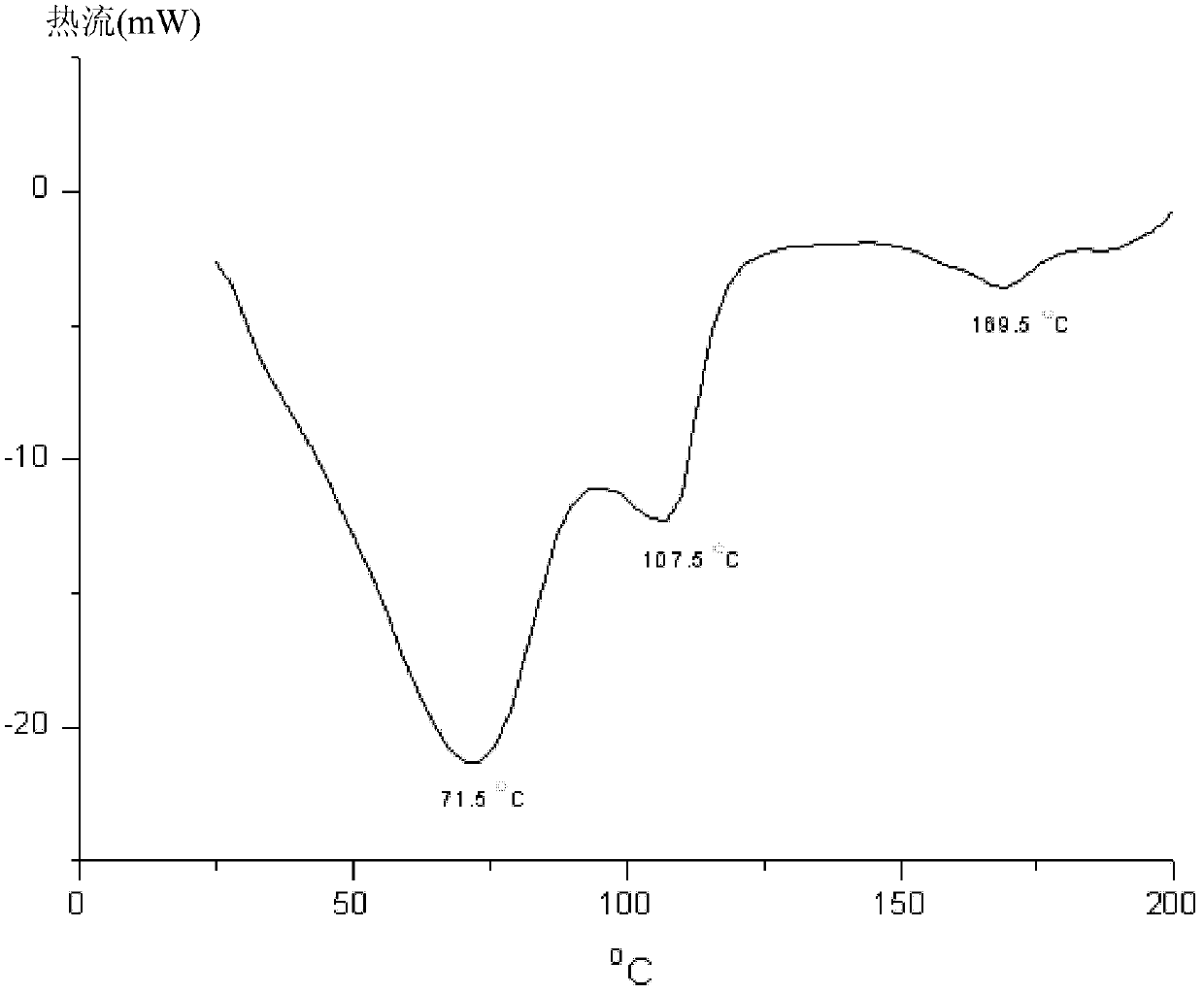
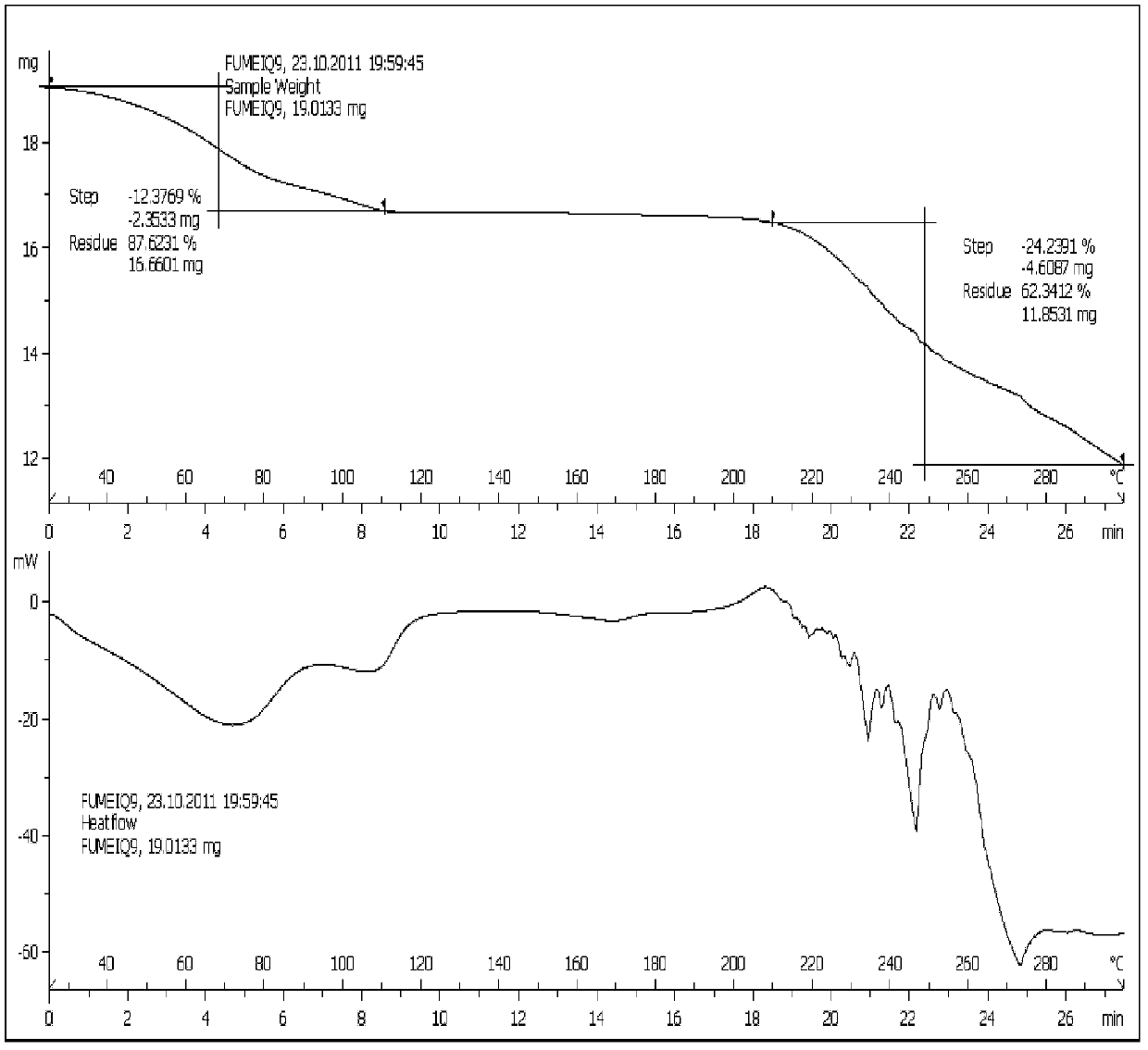
[0056] Cool 300ml of ethanol with ice water to 15°C-20°C, add 10g of tigecycline amorphous under stirring, and stir to dissolve. Concentrate with a rotary evaporator under vacuum, and control the temperature of the water bath below 25°C. When concentrated to 100-110ml, stop concentrating. Put the concentrated solution in an ice-water bath, add 100-110ml of ethyl acetate while stirring, and when the ice-water bath is stirred to about 5°C, put it in a freezer (-20°C-0) to crystallize for 10-15 hours. After filtration, the crystal was vacuum pumped for 20-24 hours at 25°C-30°C to obtain 8.20 g of the new crystal form described in this patent (purity: 99.7%, residual solvent: ethanol 7.24%, ethyl acetate 2.9%).
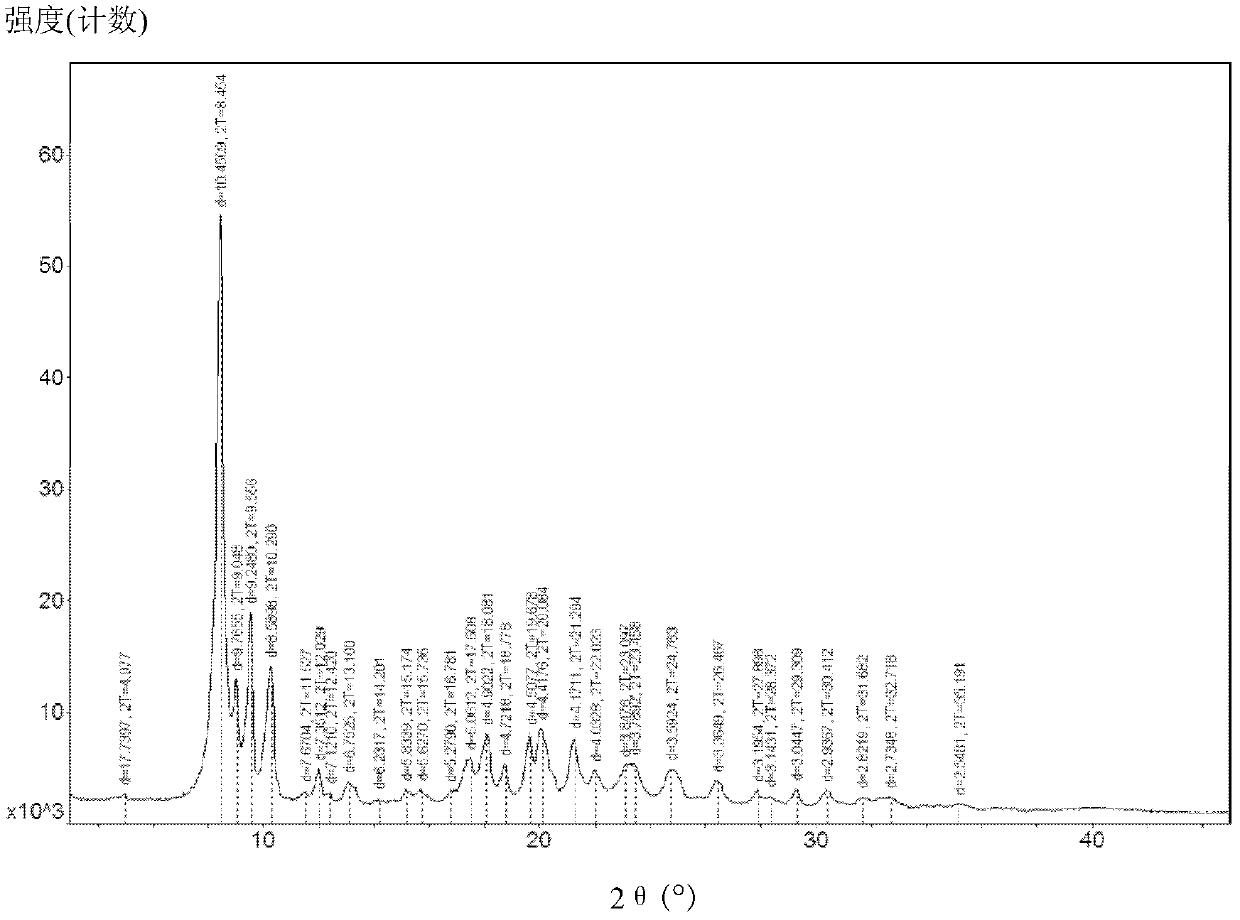
[0057] This crystal is used as a sample, and powder X-ray diffraction (hereinafter referred to as XRD) is measured to obtain figure 1 The X-ray diffraction pattern shown.
[0058] This crystallization is u...
Embodiment 2
[0060] Embodiment 2: Preparation of tigecycline crystal form
[0061] Cool 400ml of methanol with ice water to 15°C-20°C, add 10g of tigecycline amorphous under stirring, and stir to dissolve. Concentrate with a rotary evaporator under vacuum, and control the temperature of the water bath below 25°C. When concentrated to 100-110ml, stop concentrating. Put the concentrated solution in an ice-water bath, add 100-110ml of ethyl acetate while stirring, and when the ice-water bath is stirred to about 5°C, put it in a freezer (-20°C-0) to crystallize for 10-15 hours. Filtrate, vacuumize the crystals at 25°C-30°C for 20-24 hours to obtain the new crystal form described in this patent, and obtain 9.1g tigecycline crystal form (purity: 99.6%, residual solvent: methanol 0.24% , ethyl acetate 3.7%). The X-ray diffraction figure of described crystal form tigecycline is as attached figure 1 shown.
Embodiment 3
[0062] Embodiment 3: Preparation of tigecycline crystal form
[0063] Cool 300ml of isopropanol with ice water to 15°C to 20°C, add 10g of tigecycline amorphous under stirring, and stir to dissolve. Concentrate with a rotary evaporator under vacuum, and control the temperature of the water bath below 25°C. When concentrated to 100-110ml, stop concentrating. Put the concentrated solution in an ice-water bath, add 100-110ml of ethyl acetate while stirring, and when the ice-water bath is stirred to about 5°C, put it in a freezer (-20°C-0) to crystallize for 10-15 hours. After filtration, the crystals are vacuumed for 20 to 24 hours in an environment of 25°C to 30°C to obtain the new crystal form described in this patent. 8.7 g of tigecycline were obtained (purity: 99.5%, residual solvent: isopropanol 7.32%, ethyl acetate 1.7%). The X-ray diffraction figure of described crystal form tigecycline is as attached figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com