Modulators of 5-ht receptors and methods of use thereof
A technology of m-g1 and -NO2, applied in the modulator of 5-HT receptor and its application field, can solve the problems of limiting treatment efficacy, affecting patient cooperation, primary negative and cognitive symptom recognition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 42
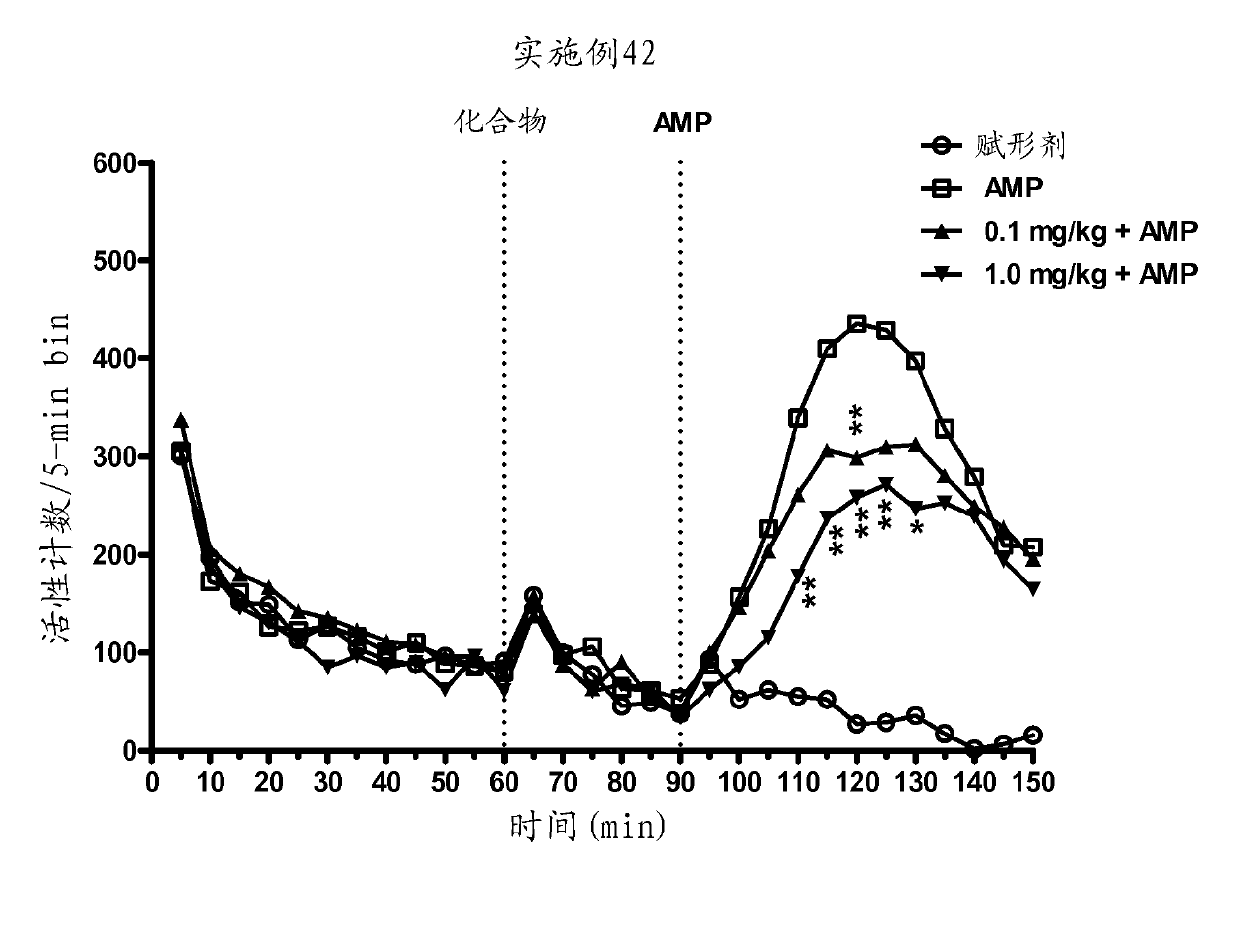
[0606] Example 42 significantly and dose-dependently attenuated AMP-induced hyperactivity (main effect F(3,29)=6.2, Pfigure 1 ).
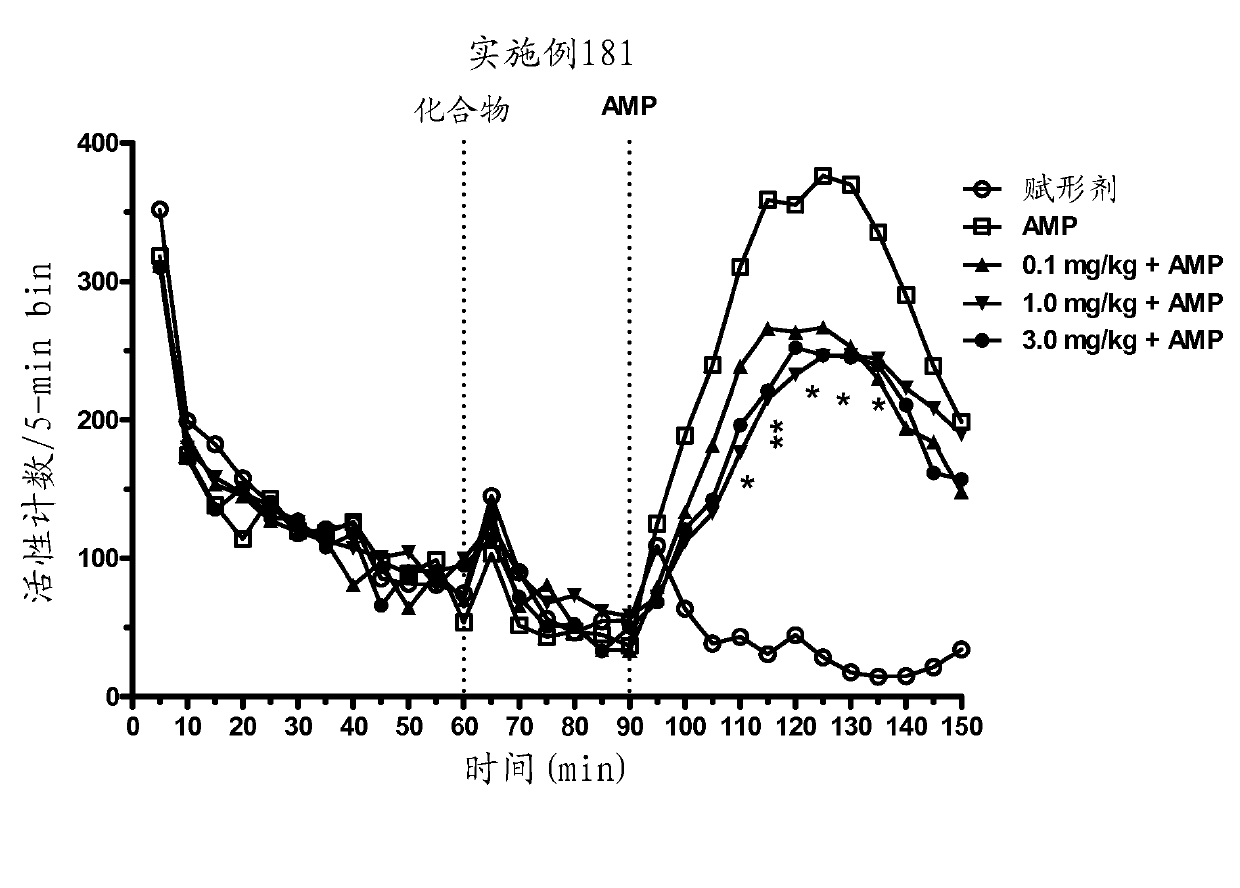
[0607] Example 181 administered to animals at 1.0 and 3.0 mg / kg before AMP significantly and in a dose-dependent manner attenuated AMP-induced hyperactivity (main effect F(7,29)=11.5, P Figure 2a ). In addition, no effect of Example 181 was seen in the spontaneous activity ( Figure 2b ).
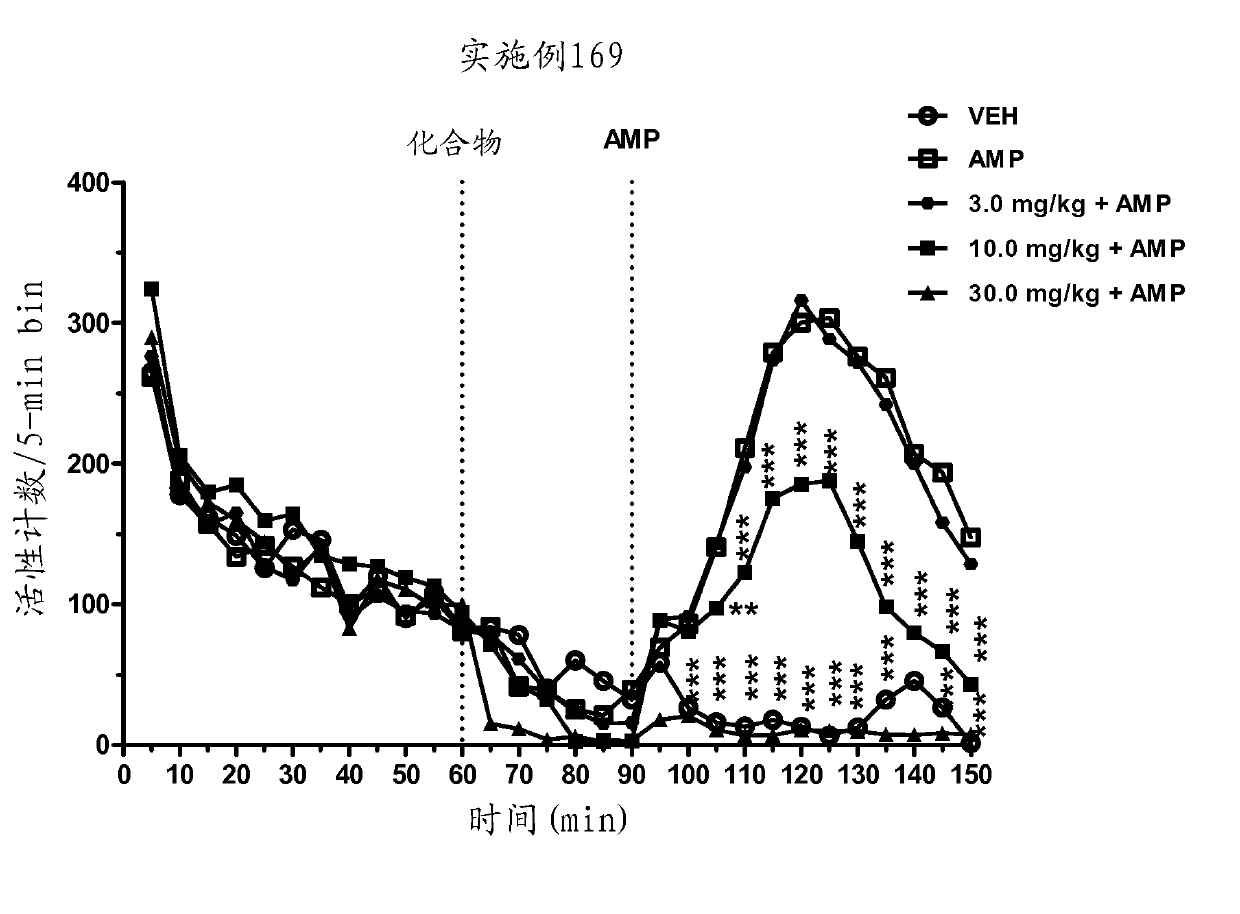
[0608] Example 169 given to animals at 10 and 30 mg / kg before AMP; significantly and in a dose-dependent manner attenuated AMP-induced hyperactivity (main effect F(7,29)=12.6, P Figure 3a ). In addition, no effect of Example 169 was seen in the spontaneous activity ( Figure 3b ).
Embodiment 2
[0609] Example 2 significantly and in a dose-dependent manner weakened PCP-induced hyperactivity (main effect F(4,26)=3.5, P Figure 4 ).
[0610] d. Methods of Using Compounds
[0611] The compound of the present invention is 5-HT 2C receptor or 5-HT 6 receptor modulator or 5-HT 2C and 5-HT 6 Modulator of both receptors. In certain embodiments of the invention, the compound of formula (I) is 5-HT 2C Agonists and partial agonists of receptors or 5-HT 6 receptor antagonists. In certain other embodiments of the invention, the compound of formula (I) is 5-HT 2C Agonists and partial agonists of receptors and 5-HT 6 receptor antagonists. Thus, such compounds are useful in the prevention or treatment of 5-HT 2C and 5-HT 6 It is of interest in disease conditions in which either or both receptors are involved. Accordingly, the present invention provides methods of preventing or treating such conditions in a subject in need thereof. A subject in need of treatment thereof ma...
Embodiment 1
[0729] 1,2,3,4,4a,5-Hexahydropyrazino[1,2-a][1,5]benzodiazepine-6(7H)-one
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com