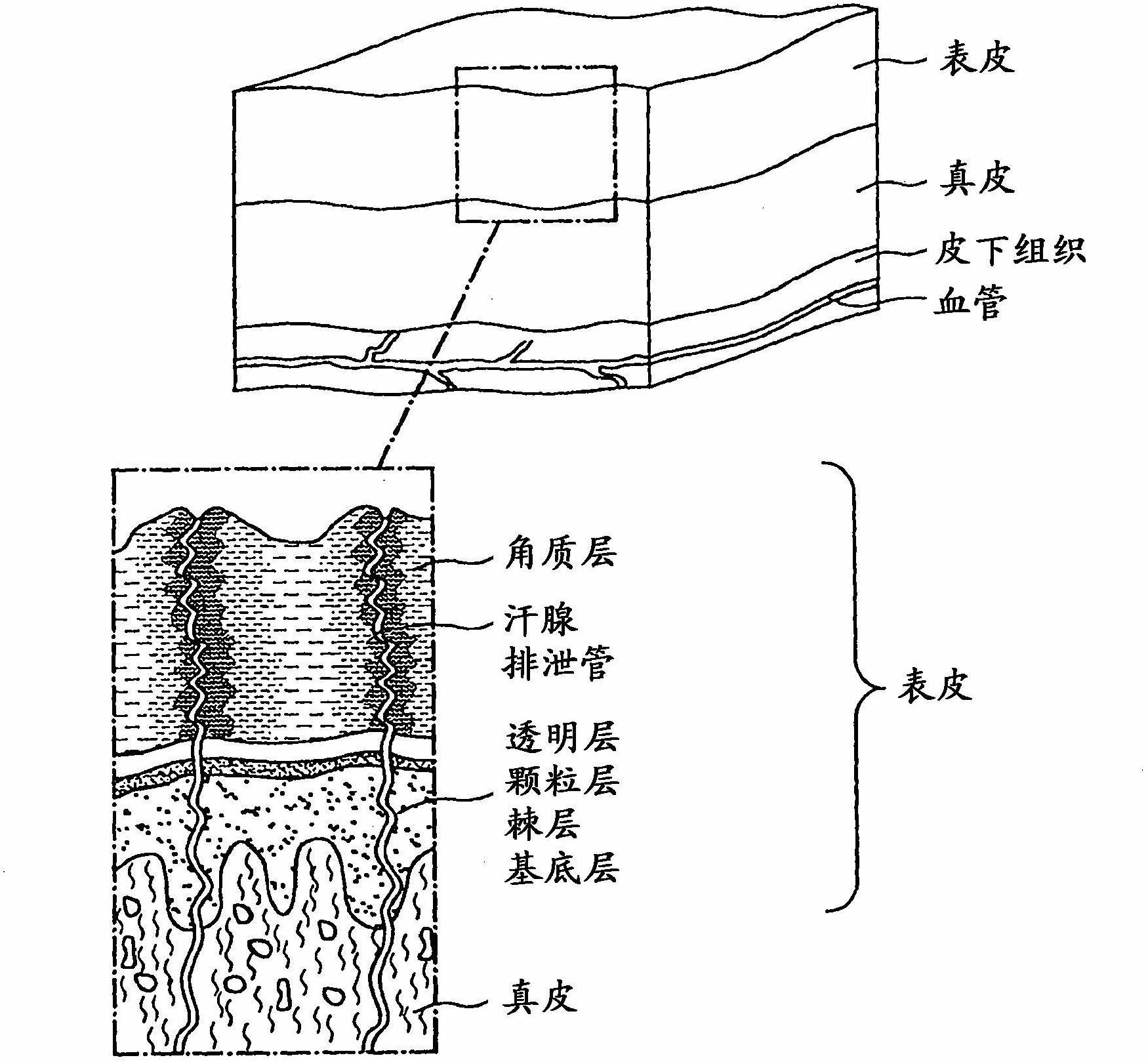
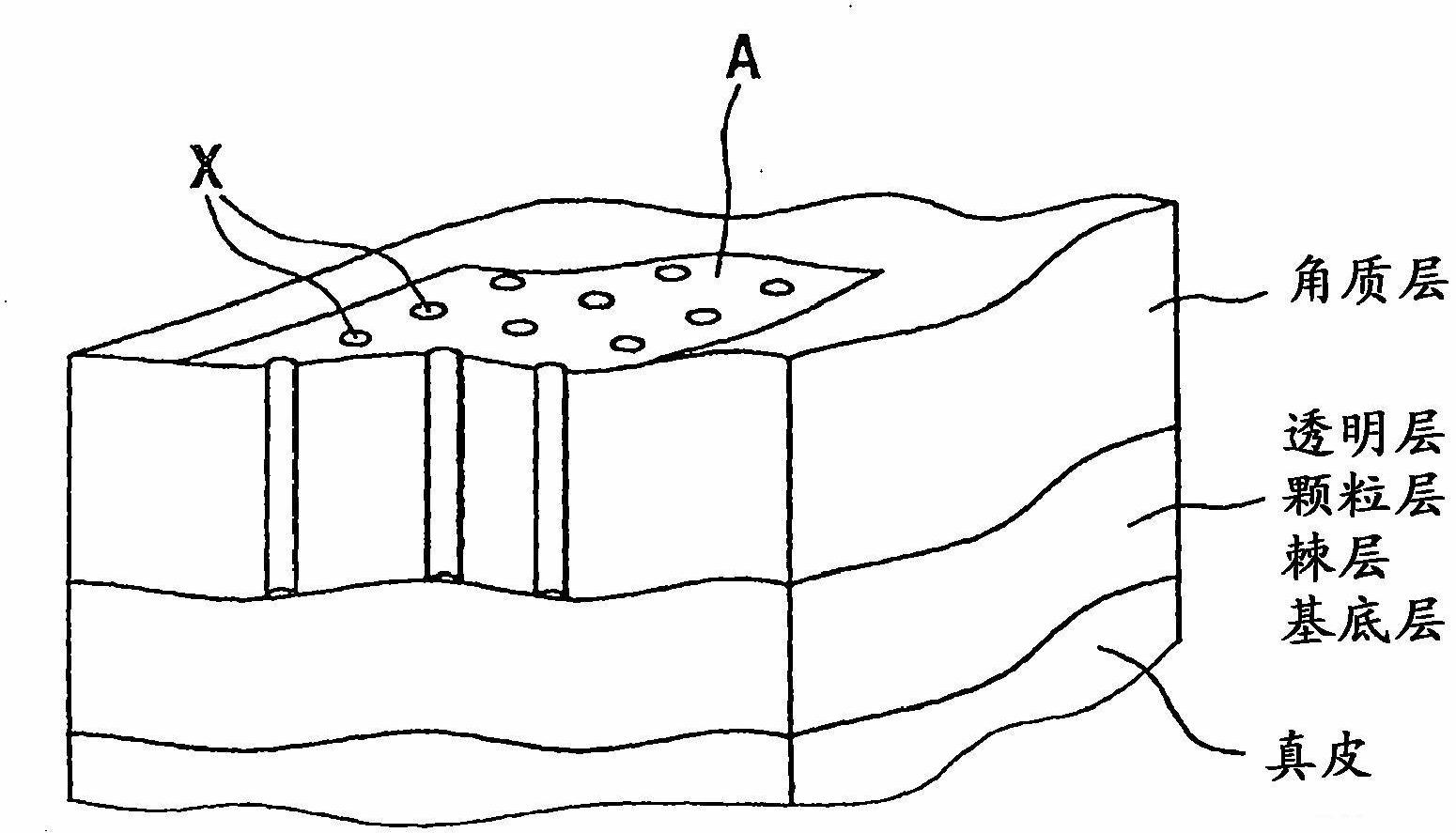
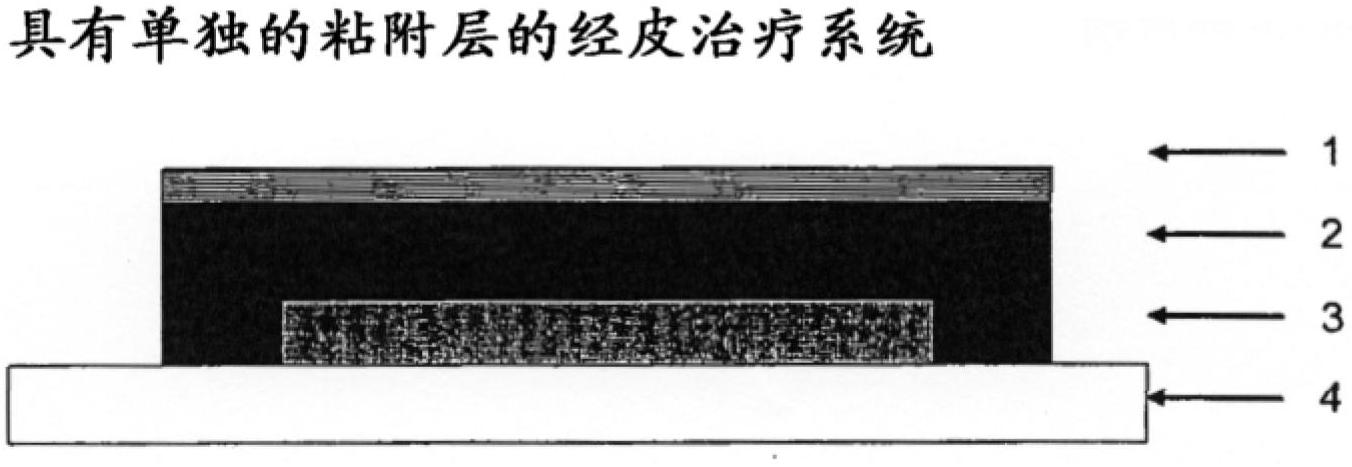
Transdermal therapeutic system for the administration of peptides
A treatment system and drug delivery technology, applied to urinary system diseases, antineoplastic drugs, drug combinations, etc., can solve problems such as injuries, infections, and difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0104] Dissolve triptorelin acetate in aqueous acetate buffer solution (pH 5.0). Mannitol was added to the solution until it assumed a 3% mannitol concentration. After adding ethanol in polyvinylpyrrolidone (Kollidon 90F) and glycerin a substance is obtained from which a film of uniform thickness is made by coating and drying solvents (water, ethanol).
[0105] The table below gives the composition of the active substance-containing layer thus obtained in the dry state.
[0106] active substance layer
Quantity (unit g)
Quantity (unit %)
triptorelin acetate
3.39
16.95
Polyvinylpyrrolidone (dissolved in ethanol)
13.47
67.35
Glycerin
2.00
10.00
Sodium acetate (trihydrate)
0.48
2.40
Acetic acid
0.12
0.60
Mannitol
0.54
2.70
purified water
...
...
the sum
20.00
100
[0107] The layer thickness is about 40 μm....
example 2
[0112] Manufactured according to the pattern of Example 1, with the difference that the loading of triptorelin acetate corresponds to 0.1 mg / cm 2 , 0.2mg / cm 2 or 0.3mg / cm 2 .
example 3
[0114] The models of the transdermal therapeutic systems produced in Examples 1 and 2 with triptorelin acetate as active substance were tested for their permeability in a Franz cell diffusion cell. The results of this test are in Figure 4 is shown in , where bovine udder skin pretreated with laser ablation was used as a model diaphragm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap