Method and apparatus for in vitro testing for medical devices
A device, fluid technology, used in the field of in vitro testing of medical devices and devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
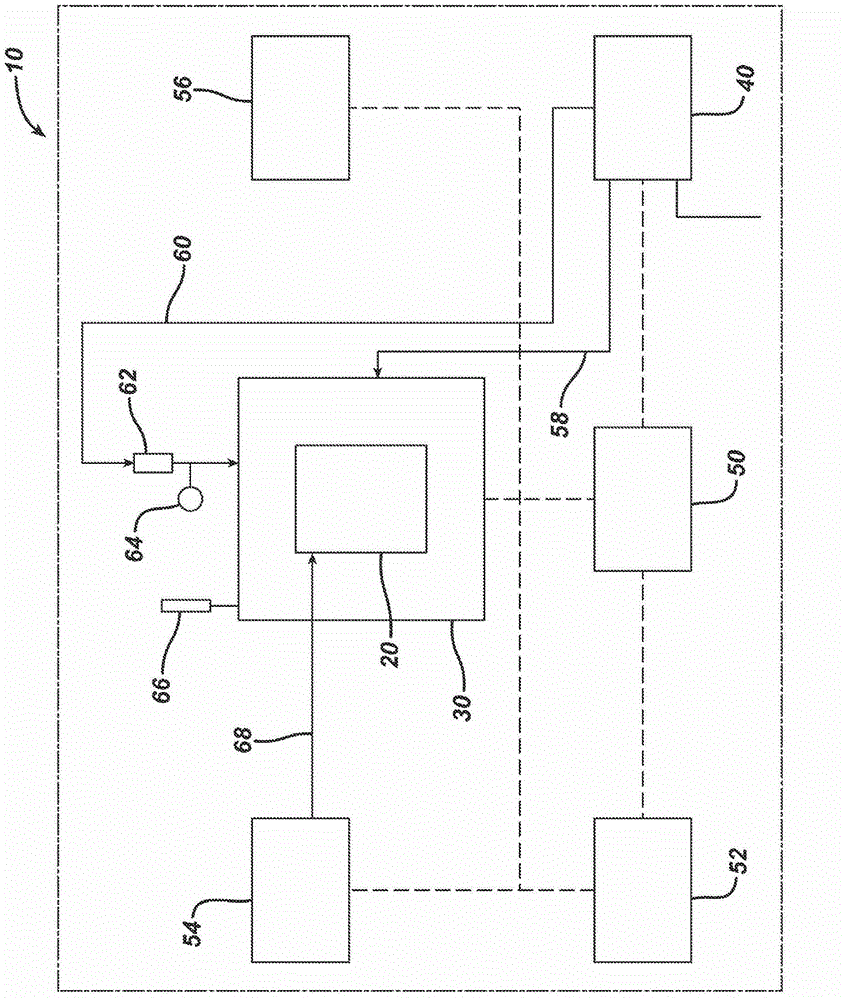
[0057] use figure 2 The diagram shown in builds an instance of SIMA for use with this example. Details and components are discussed below.
[0058] exist Figure 7 In a preferred embodiment shown schematically, the pressure chamber 30 , fluid pump 54 and GUI terminal 56 are located within a safety enclosure 70 . In addition to the safety enclosure 70 , associated compartments hold an air control panel 40 , an electrical control panel 50 , a local computer (eg PC) 52 and a printer 72 . In this example, the pressure chamber 30 is located within the enclosure 70 for safety purposes even when a safety relief valve such as 66 is used.
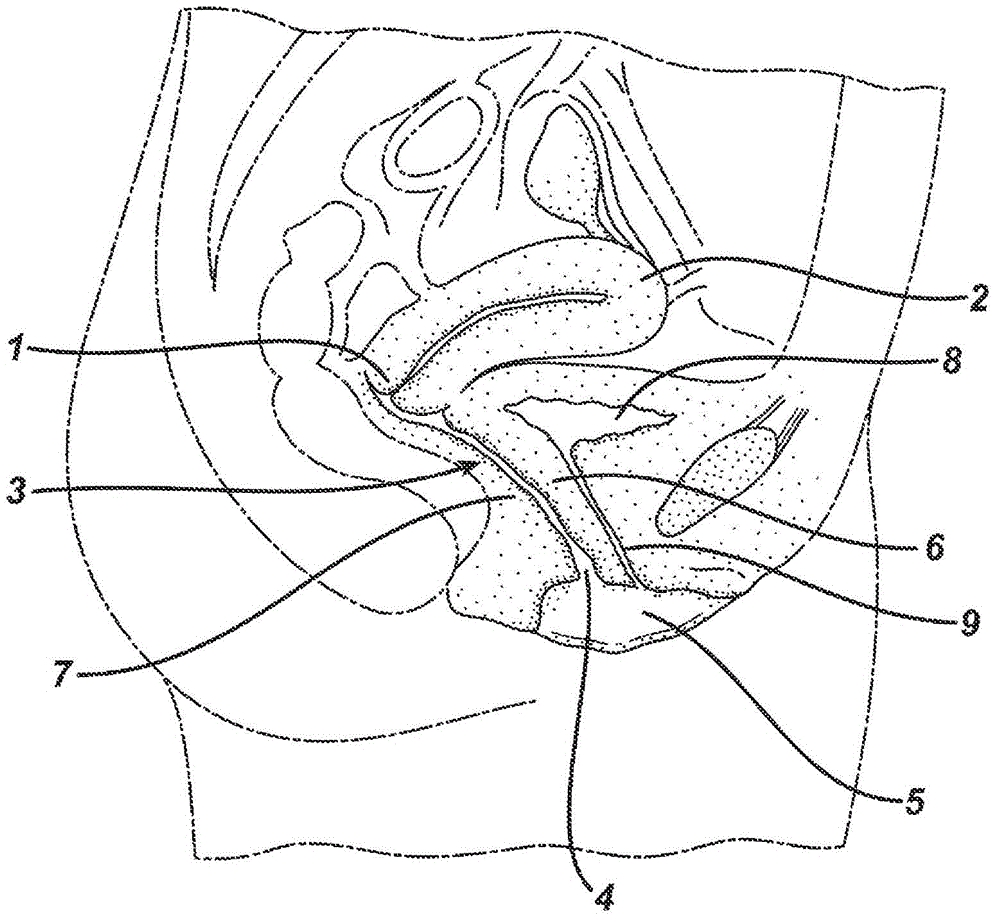
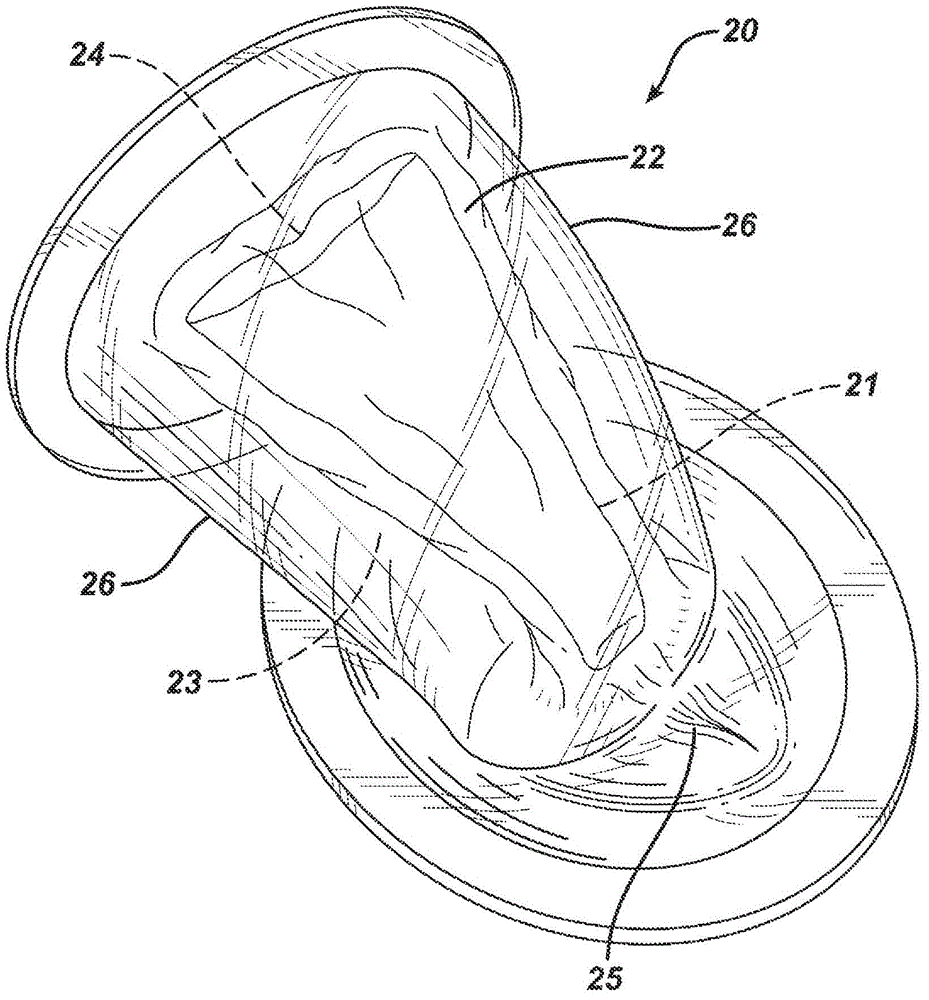
[0059] Such as figure 2 As shown in , the closed environment provided by the pressure chamber 30 enables the in vitro vaginal model 20 to withstand pressures that reproduce background body pressure and dynamic intra-abdominal pressure. The body pressure is provided by maintaining the inside of the pressure chamber 30 at a constant pressure. ...
example 2
[0074] Example 2: Manual Mode
[0075] Step A: Prepare artificial menstrual fluid according to patent publication US 20070219520 before using the device. Turn on the computer, monitor and fluid pump. Use a cotton swab to remove any menstrual fluid left in the vaginal cavity from the previous test and flush the pump with water until the hose is clean. Next, the vaginal cavity is filled with artificial menstrual fluid before the test. Calibrate the fluid pump and place the cannula in the cervical opening of the vaginal model. The box is then closed and set to the desired testing position (sitting, standing or supine). Weigh tampons in cellophane-free packaging (example: commercial tampons manufactured by McNeill Consumer Products regular absorbent tampons). Put a small amount (about 0.1 to 0.2 grams) Place on top of tampon (to facilitate insertion). Weigh the tampon with the KY gel and record the weight. Insert the tampon into the opening of the labia. In some cases...
example 3
[0079] Example 3: Automatic Mode
[0080] Repeat step A in Example 2
[0081] Step B: On the touch screen control element, press the "automatic mode" of operation. This mode of operation enables the user to re-run a previously established "recipe" or enter parameters for a new recipe. As used herein, the term "prescription" shall refer to a combination of pressure (body pressure, intra-abdominal pressure), time sequence, and flow characteristics (these all occur at preset time intervals). If this is a first time prescription, the user enters the relevant information and creates a prescription name or number, then saves. For example, enter the conditions of step B in manual mode and save as a new recipe. Enter parameters such as body pressure and step length (how long in seconds you want to run the entered body pressure). Similarly, select the cough pressure prescription, which includes the cough pressure (in cm H 2 O), cough time (in seconds), full cycle time (in seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com