Inula wissmannian extracts, preparation for same, and application of extracts in preparation of anti-inflammatory medicine
A kind of technology of Yangchuan and extract, which is applied in the direction of anti-inflammatory agent, drug combination, antipyretic, etc., can solve the problem that there is no pharmacological activity of chemical constituents of southern Yunnan Yangchuan, and no preparation of southern Yunnan Yangchuan extract is known. methods, uses and other issues to achieve the effect of great clinical application value and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
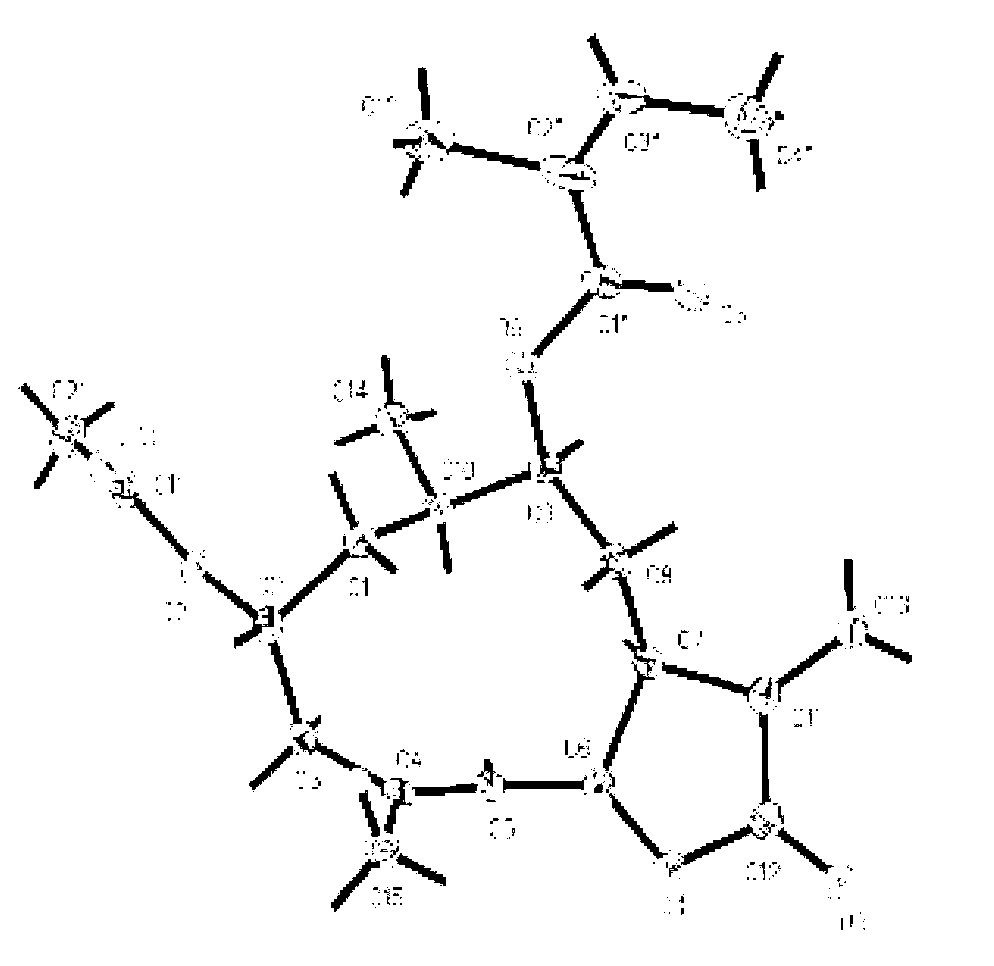
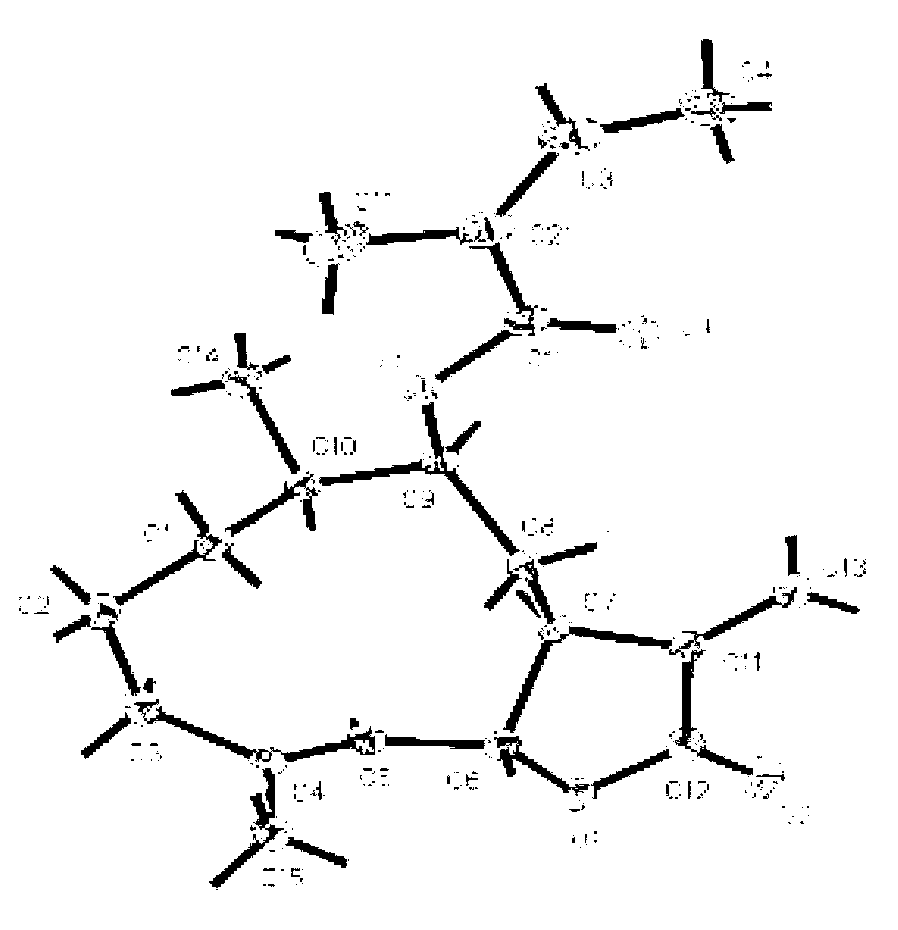
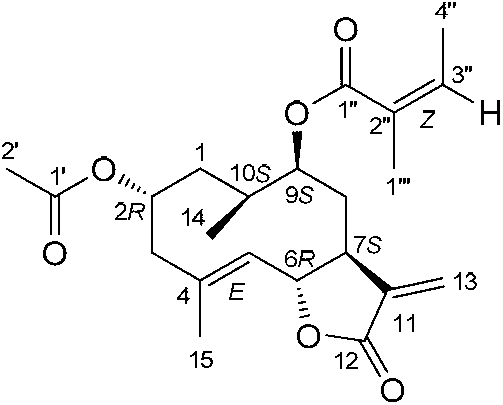
[0038] The preparation of embodiment 1 southern yunnan fennelide A, B, C, D, E and squirrel
[0039] After drying and pulverizing the whole herb of South Yunnan sheep ear chrysanthemum (10.0kg), it was cold soaked and extracted with 95% ethanol (30L) at room temperature 25°C for 4 times (24, 12, 12, 12h), the solvent was recovered, and concentrated to dryness without water soaking. cream (631.4g); the total extract was suspended in 1.5L water and extracted 3 times with 5L petroleum ether, dichloromethane and ethyl acetate successively, and the solvent was recovered under reduced pressure to obtain petroleum ether fraction (133.2g), dichloromethane Methane fractions (179.6 g) and ethyl acetate fractions (28.1 g).
[0040] Put 120 g of the dichloromethane part of the southern yunnan chrysanthemum on a normal-phase silica gel column, and use dichloromethane-methanol (100% dichloromethane, 100:1, 50:1, 20:1, 10:1, 5: 1 and 100% methanol) gradient elution, the eluent was detected ...
Embodiment 2
[0057] Example 2 Southern Diannan Fructus A, B, C, D, E and Fructus Inhibition of LPS-Induced NO Production Activity of RAW264.7
[0058] (1) Sample configuration
[0059] The southern Yunnan fechinolactones A, B, C, D, and E prepared in Example 1, fecinolactone and the positive control drug aminoguanidine (Sigma-Aldrich, purity>98.0%)) were treated with DMSO (dimethyl After dissolved in sulfoxide) (Merck), add PBS (-) (phosphate buffer saline) to make a 10mM solution, and further dilute to 0, 0.1, 0.5, 5, 20μM gradient concentration samples. 10 μg / mL of LPS (Lipopolysaccharides, lipopolysaccharide, sigma, Cat.L-2880) was used as the inducer.
[0060] (2) Experimental method
[0061] Mouse macrophage RAW264.7 (purchased from Cell Resource Center of Shanghai Academy of Biological Sciences) at 37°C, 5% CO 2Routinely cultivated in DMEM medium in the incubator. During the experiment, 1μL / mL LPS (lipopolysaccharide) (dissolved in distilled water) was added to 100mL with a conce...
Embodiment 3
[0068] Example 3 Anti-Xylene-induced Inflammation Activity of Southern Diannan Fructus A, B, C, D, E and Fructus Fructus
[0069] (1) Sample configuration
[0070] The total extract of southern yunnanensis obtained in Example 1, the petroleum ether part, the dichloromethane part, the ethyl acetate part, and the southern dianthenia lactones A, B, C, D, E, and sheep's ear Esters, were prepared with 0.5% Tween-80 (Tween-80) solution to the required concentration of the suspension, set aside. Aspirin tablets (Sigma-Aldrich Company) were prepared into a 200 mg / kg suspension before use as a positive control. The administration volume of each group was 20mL / kg, administered orally.
[0071] (2) Experimental method
[0072] 220 Kunming mice, male, 18-22 g, were randomly divided into 22 groups, 10 in each group. A group of model controls, a group of positive controls and 20 groups of drug administration groups were set up. After the mice were purchased and adapted to the environme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com