Closterovirus-based nucleic acid molecules and uses thereof
A technology of nucleic acid molecules and viruses, applied in the direction of genetic engineering, the use of vectors to introduce foreign genetic material, peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1. Construction of BYV-based launch vectors for monoclonal antibody expression
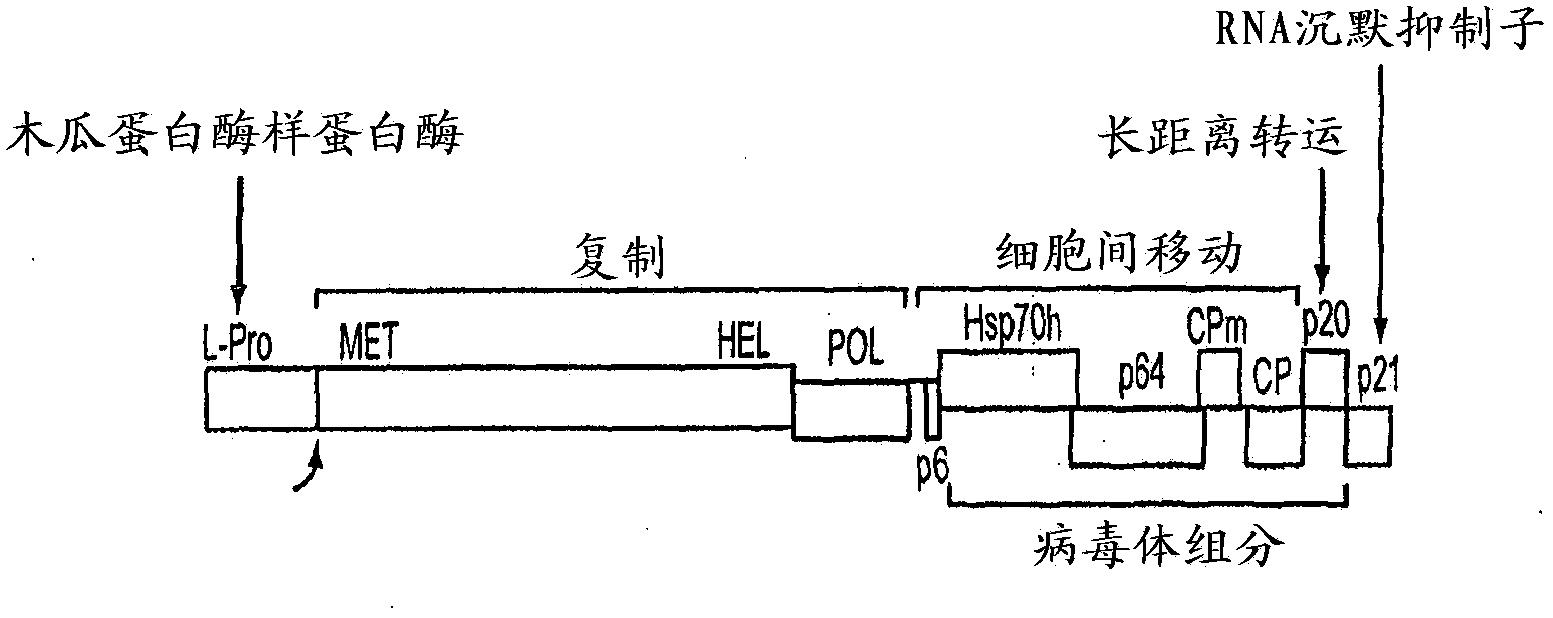
[0076] A BYV-based launch vector ( figure 2 ). The BYV launch vector was used as a vector to express two ORFs, Hc and Lc, of mAb against PA of anthrax. To clone two foreign genes (Lc and Hc of anti-PA mAb), a multiple cloning site (MCS) was introduced in the BYV genome between CPm and CP coding sequence (SEQ ID NO: 1, FIG. 12 ). In addition to its own BamHI restriction site, MCS also contains five restriction sites PacI / AscI / BsrGI / NheI / FseI. After insertion into the MCS, two heterologous clolovirus CP promoters were introduced into the BYV genome: the GLRaV2 CP promoter and the BYSV CP promoter ( figure 2 ). Thus, the Hc (SEQ ID NO: 9; Figure 16 ) and Lc (SEQ ID NO: 7; Figure 15 ) sequences were cloned under the control of the BYV CP and GLRaV2CP promoters, respectively. Meanwhile, BYV CP promoter drives BYV CP ( figure 2 ). The resulting construct pCB-BYV-PA-HcLc was ...
Embodiment 2
[0077] Example 2. Expression of anti-PA mAb in leaves of systemic BYV infection
[0078] To confirm the stability of the virus and the expression and assembly of the anti-PA mAb, Agrobacterium harboring BYV vectors encoding Hc and Lc of the anti-PA mAb and Agrobacterium harboring the Hc and Lc encoding anti-PA mAbs from Japanese radish mosaic virus ( Agrobacterium culture of the binary vector of the silencing suppressor P1HcPro of Turnip mosaic virus, at 1.0:0.2OD 600 ratio of 5-week-old N. benthamiana leaves were artificially co-infiltrated (Kasschau et al., 2003). After 30dpi, systemic symptoms of BYV infection were observed ( Figure 3A ). Specifically, infected leaves showed clearing veins as a systemic symptom. Samples were taken from systemically infected leaves at 30, 32, 34, 36 and 39 dpi.
[0079] To show the expression of Lc and Hc of the anti-PA mAb, Western blot analysis ( Figure 3B ). To assess the expression levels of Hc and Lc, goat anti-human Hc and Lc a...
Embodiment 3
[0080] Embodiment 3. Construction of the BYV minireplicon based on T-DNA
[0081] In order to reduce production time and increase antibody expression levels, a T-DNA-based BYV minireplicon (miniBYV) was engineered by removing all non-essential genes for viral replication from the BYV-based launch vector described in Example 1 ( figure 1 , 2 and 4). Using the native BamHI restriction site, the same MCS as that inserted into the full-length BYV-based vector of Example 1 was introduced into the miniBYV replicon (SEQ ID NO: 2; FIG. 13 ) ( figure 2 and 4 ). This strategy allows the expression of two foreign genes from a single miniBYV replicon using the subunit promoters of heterologous cloloviruses. In addition, the promoters BYV CP promoter and GLRaV2CP promoter of two long clodoviruses were introduced to drive the Hc of anti-PA mAb respectively (SEQ ID NO: 9; Figure 16 ) and Lc (SEQ ID NO: 7; Figure 15 ).
[0082] To prevent splicing and enhance the efficiency of viral...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap