Honokiol nanoparticles and preparation method thereof
A honokiol and nanoparticle technology, applied in the field of pharmaceutical preparations, can solve the problems of poor water solubility, low oral bioavailability, and low drug loading, and achieve the effects of facilitating treatment, improving tissue distribution in the body, and improving drug distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
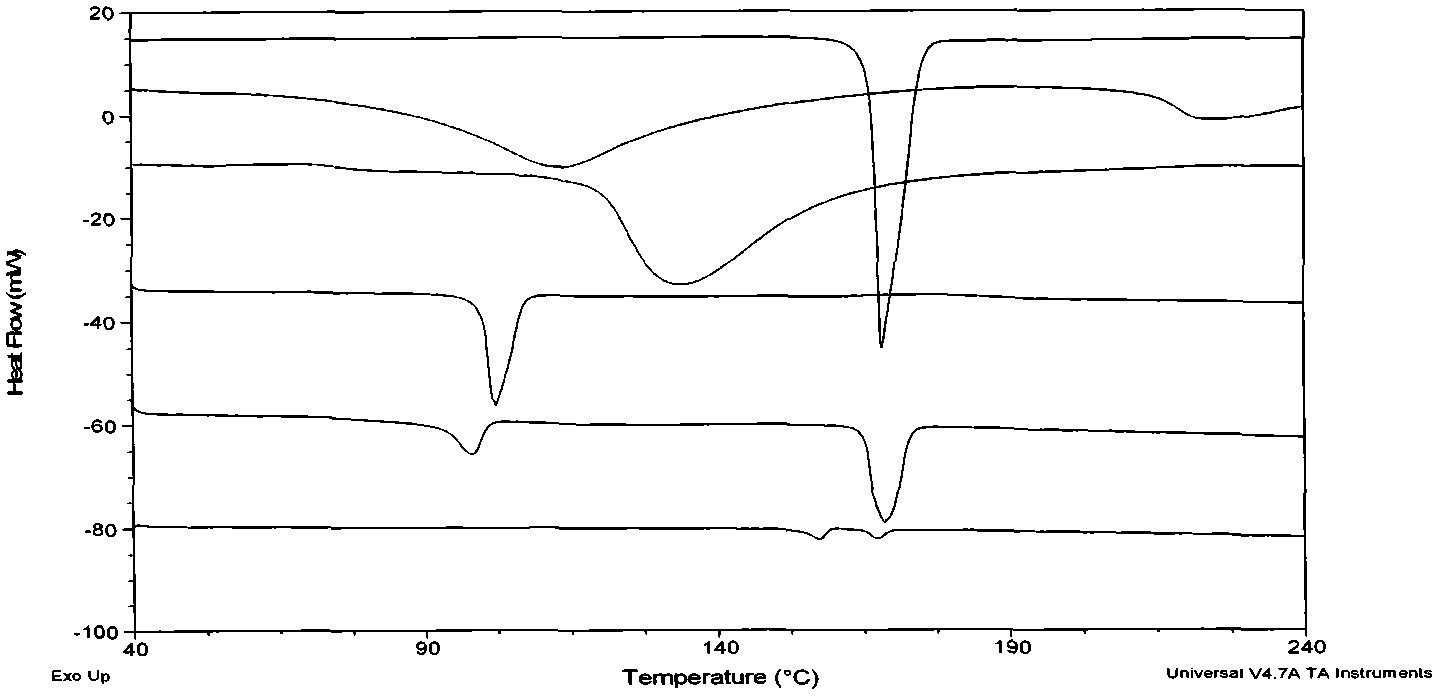
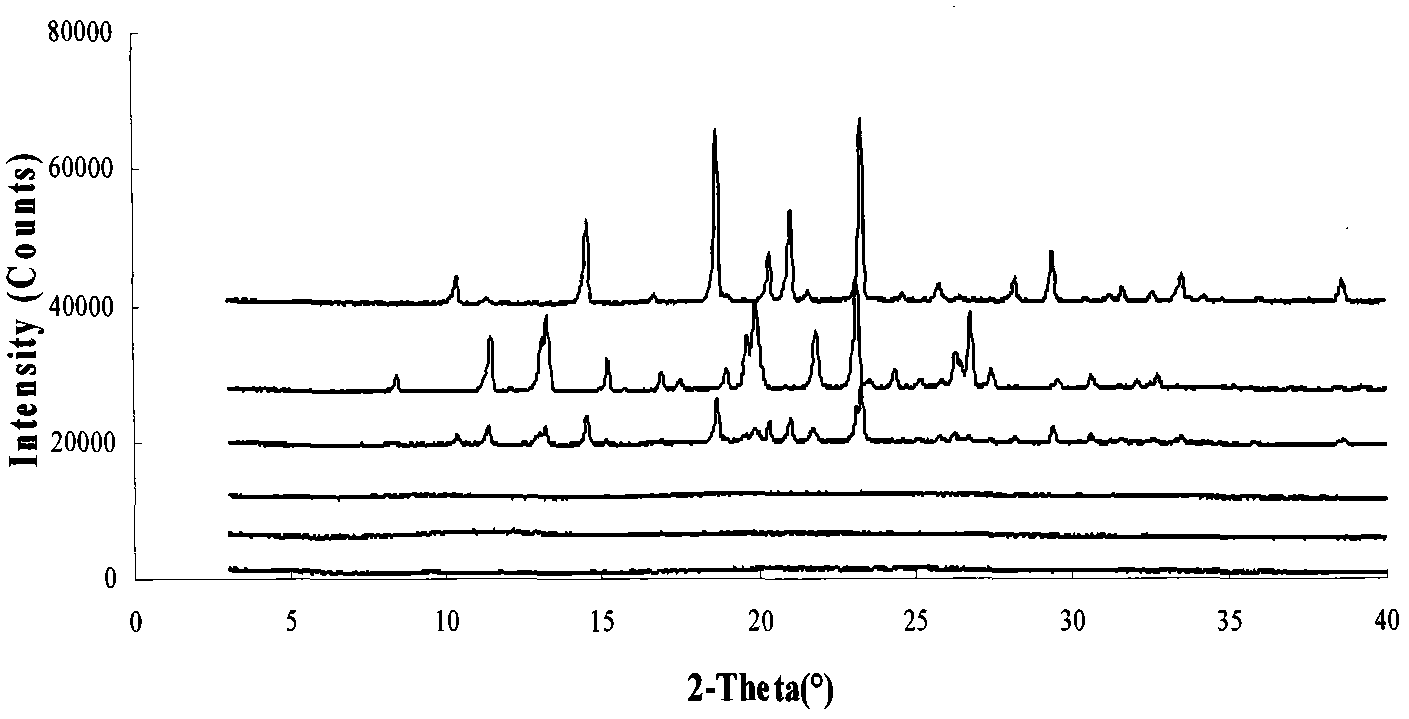
Image
Examples
Embodiment 1
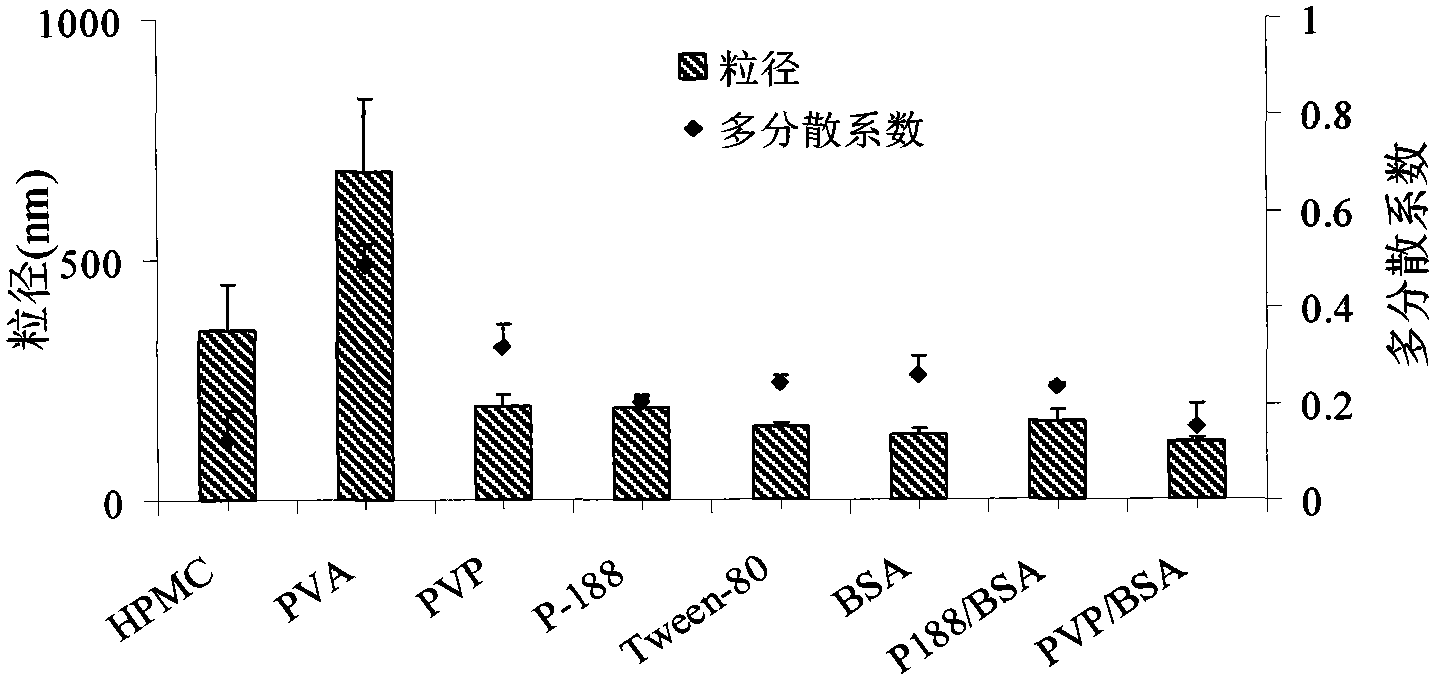
[0029] Add 100mg of hydroxypropyl methylcellulose into 20mL of water, stir to dissolve it completely, completely dissolve 40mg of honokiol in 2mL of acetone, slowly add this solution dropwise into 20mL of aqueous solution with a syringe, 200W ultrasonic for 10min, The temperature of the water bath was room temperature. Obtain honokiol initial suspension, remove acetone by rotary evaporation under reduced pressure, namely obtain honokiol nanoparticles, the average particle diameter is 353nm ( figure 1 ).
Embodiment 2
[0031] Add 200mg of Poloxamer P188 into 20mL of water, stir to dissolve it completely, completely dissolve 100mg of honokiol in 3mL of acetone, slowly add this solution dropwise into 20mL of aqueous solution with a syringe, stir for 10min, and the temperature of the water bath is room temperature . Obtain the initial suspension of honokiol, remove the acetone by rotary evaporation under reduced pressure, obtain honokiol nanoparticles, and the average particle diameter is 189nm ( figure 1 ).
Embodiment 3
[0033] Add 40mg of Tween-80 into 20mL of water, stir to dissolve it completely, completely dissolve 40mg of honokiol in 1mL of acetone, quickly drop this solution into 20mL of aqueous solution with a syringe, 250W ultrasonic for 8min, and the temperature of the water bath is 0°C. Obtain honokiol initial suspension, remove acetone by rotary evaporation under reduced pressure, namely obtain honokiol nanoparticles, the average particle diameter is 151nm ( figure 1 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com