The application of blue calyx fragrant tea in the preparation of anti-acute lung injury medicine
A technology for the preparation of anti-acute lung injury medicines for the preparation of the anti-acute lung injury of the blue calyx fragrant tea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
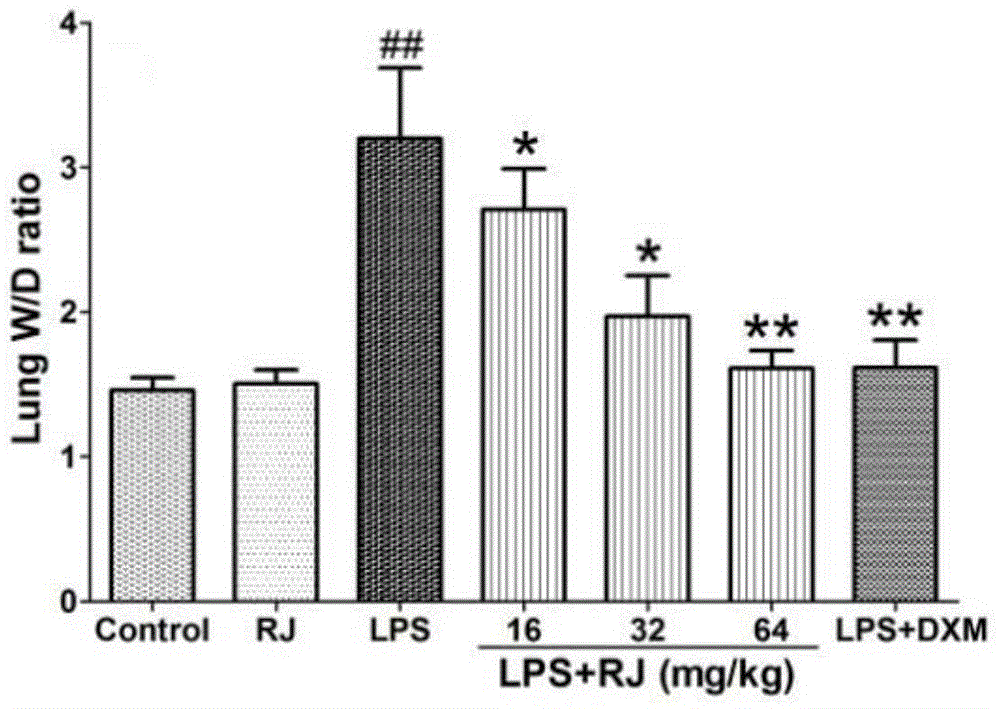
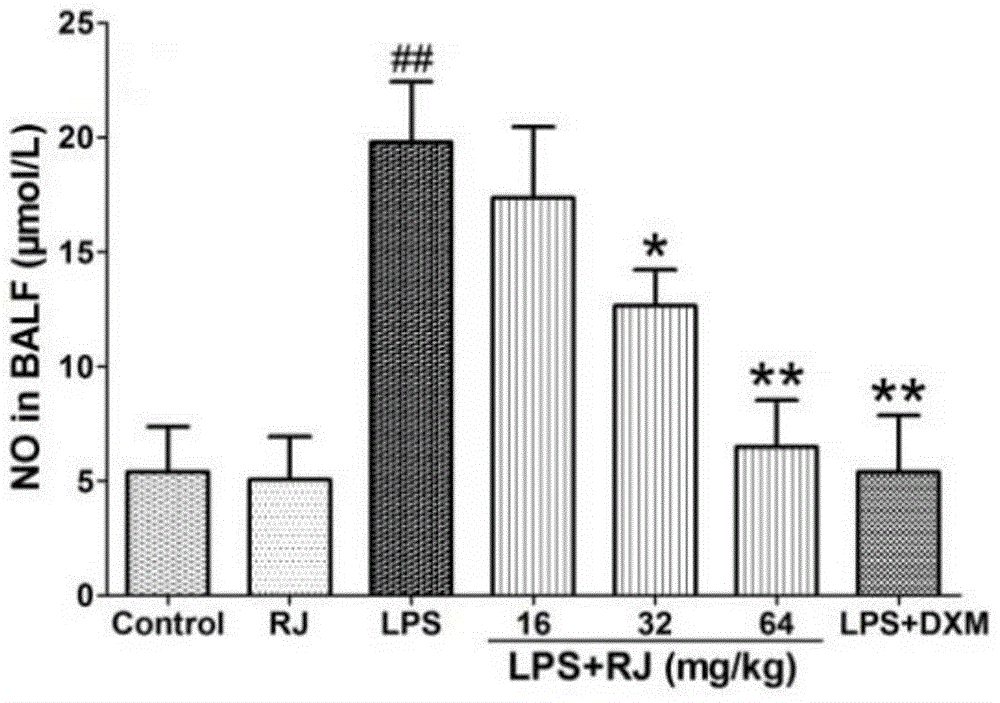
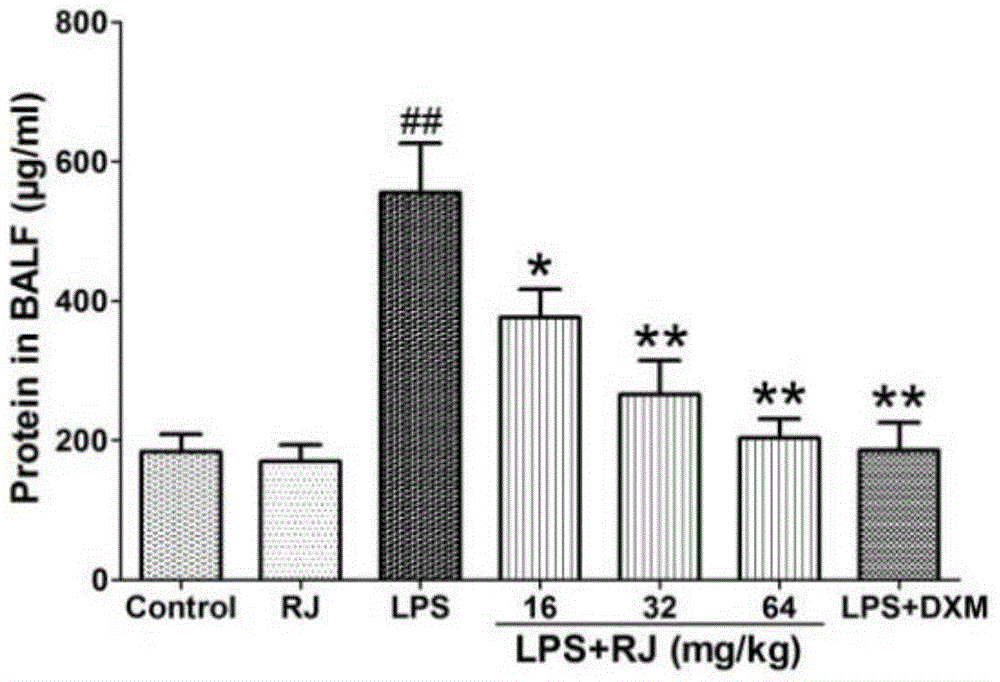
[0033] Anti-acute lung injury experiment of the ethanol extract (RJ) of the calyx calyx
[0034] 1. Experimental medicines and reagents: ethanol extract of Lemonia japonica; 0.5% sodium carboxymethylcellulose; lipopolysaccharide (LPS), Sigma-Aldrich Company of the United States; control drug, dexamethasone acetate tablets, Zhejiang Xianju Pharmaceutical Co., Ltd.; distilled water; normal saline; 20% urethane-normal saline; 4% formalin, H-E staining solution, etc.
[0035]2. Experimental mice: Kunming mice, male, weighing 24-28 grams, provided by the Experimental Animal Center of Soochow University, experimental animal production license: XCYK (Su) 2002-0008. The experimental animals were raised in an environment with a temperature of 24±1° C. and a relative humidity of 40% to 80%, with free access to food and water. Before the experiment, they were adaptively fed for 7 days.
[0036] 3. Other materials: ELISA kit, Shanghai Chuanfu Trading Co., Ltd.; nitric oxide (NO), BCA, s...
Embodiment 2
[0053] Anti-acute lung injury experiment of macroporous resin fractions (RJFs) of the ethanol extract of C.
[0054] The experimental drugs and reagents, experimental mice, other equipment, test methods and experimental data used in this example are all processed in the same manner as in Implementation 1. Among them, the ethanol extract (RJ) of R. japonica was changed to the macroporous resin fraction (RJFs) of the ethanol extract of R. japonica (RJFs), and the dosage of the control group was changed to 25.6mg / kg. The samples of large and medium The administration doses of the low-dose and low-dose groups were 6.4 mg / kg, 12.8 mg / kg, and 25.6 mg / kg, respectively.
[0055] After the experimental data was processed, the indicators for evaluating acute lung injury were compared among the experimental groups, and the results were as follows: Figure 10-18 shown.
[0056] From Figure 10-12 It can be seen that compared with the blank group, the W / D weight ratio, NO (nitric oxide)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com