A chiral imine-containing pyridine oxazoline compound and its preparation method
A technology for imine pyridine oxazoline and compounds, which is applied in the field of synthesis of compounds containing imine pyridine oxazoline, which can solve problems such as asymmetry and achieve high chemical conversion rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0065] Embodiment: Amine formula (3) is commercially available, and 2-bromo-6-acylpyridine formula (2) is according to literature (Ruifa Zong, Dong Wang, Richard Hammitt, and RandolphP.Thummel.J.Org.Chem. , 2006,71,167) prepared. Oxazoline ring formula (5) according to literature ((a) Bandyopadhyay, S.; Zhou, W.; Breslow, R.Org. Lett.2007, 9, 1009; (b) Levine, M.; Kenesky, C.S.; Zheng, S.; Quinn, J.; Breslow, R. Tetrahedron Lett. 2008, 49, 5746.) Preparation.
[0066] Preparation of 2-bromo-6-iminopyridine formula (4)
example A1
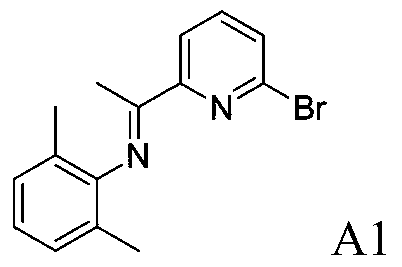
[0067] Example A1: Preparation of 2-bromo-6-iminopyridine A1
[0068]
[0069] 2,6-Dimethylaniline (2.9083g, 24mmol, 1.2equiv) and 2-bromo-6-acetylpyridine (4.0006g, 20mmol, 1.0equiv) were dissolved in 50mL of toluene, p-toluenesulfonic acid (0.0760g, 0.4mmol, 2mol%), reacted for 24h, and recrystallized from ethanol to obtain 4.7901g (15.8mmol, 79%) of 2-bromo-6-iminopyridine A1.
[0070] 1 H NMR (400MHz, CDCl 3 )δ8.33(d, J=7.7Hz, 1H), 7.64(t, J=7.7Hz, 1H), 7.56(d, J=7.7Hz, 1H), 7.06(d, J=7.5Hz, 2H) ,6.93(t,J=7.5Hz,1H),2.15(s,3H),2.01(s,6H). 13 CNMR (100MHz, CDCl 3 )δ166.15, 157.44, 148.44, 140.97, 138.74, 129.25, 127.95, 125.25, 123.26, 120.03, 17.90, 16.63.
example A2
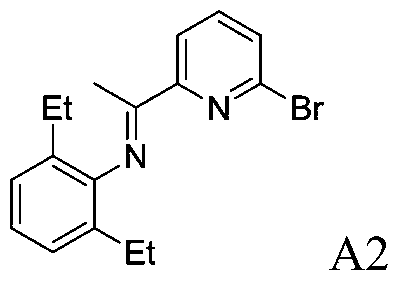
[0071] Example A2: Preparation of 2-bromo-6-iminopyridine A2
[0072]
[0073] 2,6-Diethylaniline (3.5815g, 24mmol, 1.2equiv) and 2-bromo-6-acetylpyridine (4.0006g, 20mmol, 1.0equiv) were dissolved in 50mL of toluene, p-toluenesulfonic acid (0.0760g, 0.4mmol, 2mol%) catalyzed, reacted for 24h, recrystallized from ethanol to obtain 5.5341g (16.7mmol, 84%) 2-bromo-6-iminopyridine A2.
[0074] 1 H NMR (400MHz, CDCl 3 )δ8.32(d, J=7.7Hz, 1H), 7.65(t, J=7.7Hz, 1H), 7.57(d, J=7.7Hz, 1H), 7.25–6.98(m, 3H), 2.34( m, 4H), 2.17(s, 3H), 1.12(t, J=7.5Hz, 6H). 13 C NMR (100MHz, CDCl 3 )δ165.90,157.46,147.47,140.99,138.75,131.05,129.21,125.99,123.55,119.99,24.57,16.96,13.70.calcd for m / z C 17 h 19 BrN 2 330.0732,found m / z330.0735.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com