A kind of platinum (ii) complex with chiral bicyclic diamine as carrier ligand and its preparation method and application
A complex and chiral technology, applied in platinum group organic compounds, platinum organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve problems such as treatment failure of cisplatin drugs , to achieve the effect of overcoming cisplatin resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of Ligands LR and LS.
[0037] (1) Synthesis of bicyclo[2,2,2]octane-2-alkene-7,8-trans diacid chloride
[0038] Add 4.1g (50mmol) of 1,3-cyclohexadiene into a 50mL single-necked round-bottomed flask, and slowly add 8.8g (55mmol) of fumaryl chloride dropwise at 0°C. After 12 hours of reaction, the target product bicyclo[2,2,2]octane-2-alkene-7,8-trans-diacid chloride was obtained.
[0039] (2) Synthesis of bicyclo[2,2,2]octane-2-alkene-7,8-trans diamine
[0040] 13.0g (200mmol) NaN 3and 50mL of water into a 250mL single-necked round bottom flask. Slowly add 100 mL of toluene solution of bicyclo[2,2,2]octane-2-alkene-7,8-trans-diacid chloride, the step product, into the reaction liquid at 0°C, and stir the reaction at this temperature for 3 h . After the reaction was completed, the reaction liquid was extracted with toluene (3×150 mL), and the organic phase was collected and dried over anhydrous sodium sulfate. Add the dried toluene solution ...
Embodiment 2
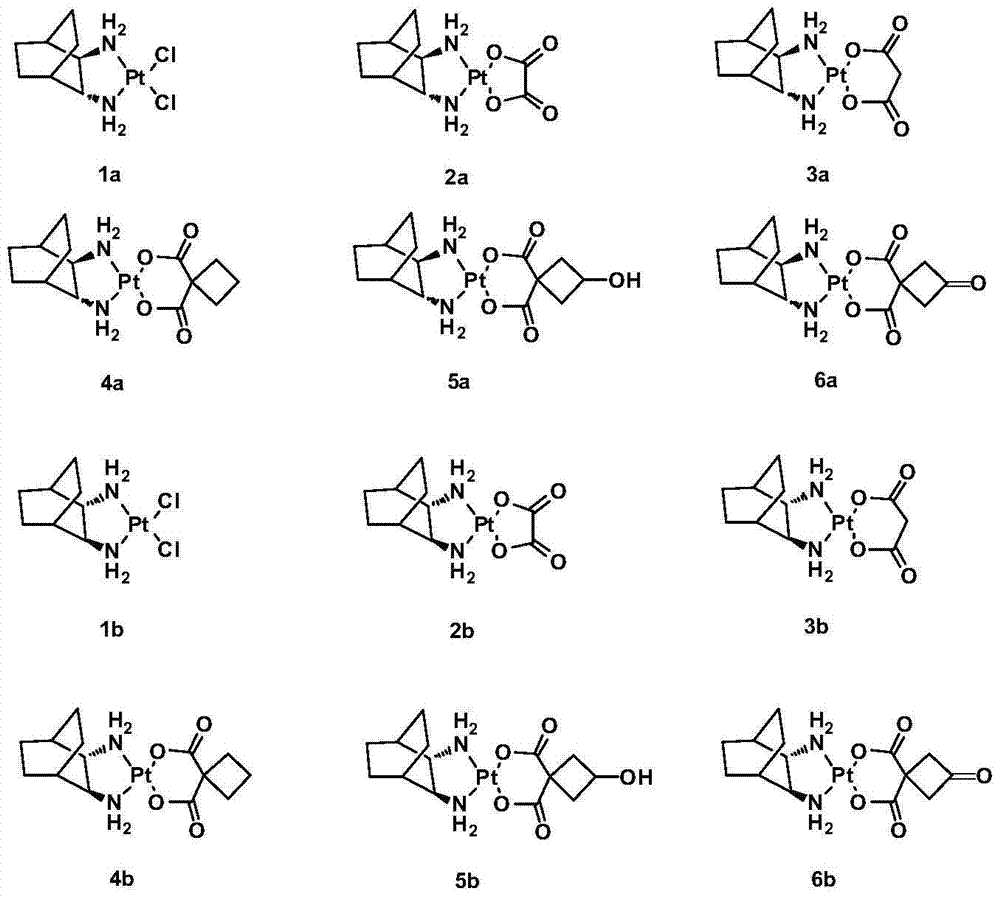
[0048] Example 2: Synthesis of complex 1a.
[0049] Dissolve 2.50g (6.00mmol) potassium tetrachloroplatinate and 0.84g (6.00mmol) LR in 50mL deionized water, stir and react at 30°C for 12 hours in the dark, a bright yellow precipitate is formed, filter it, wash it repeatedly with water, and dry it brightly Yellow solid powder 2.43g, yield 89%. Elem anal. Calcd for C 8 h 16 Cl 2 N 2 Pt: C, 23.66; H, 3.98; N, 6.90. Found: C, 23.41; H, 4.25; N, 7.28. IR (KBr, cm -1 ): 3194, 2939, 2916, 2869, 477, 411; 1 H NMR (300MHz, d 6 -DMSO): δ1.19-1.77 (m, 8H, CH 2 of LR), 1.80-1.91 (m, 2H, CH of LR), 2.53(d, 2H, J=5.2Hz, 2×NH 2 CH ), 5.80-6.51 (m, 4H, 2×CH NH 2 ); 13 C NMR(d 6 -DMSO / TMS, ppm): δ19.15, 19.40, 25.99, 26.03, 31.24, 63.10, 64.07.
Embodiment 3
[0050] Example 3: Synthesis of Complex 2a.
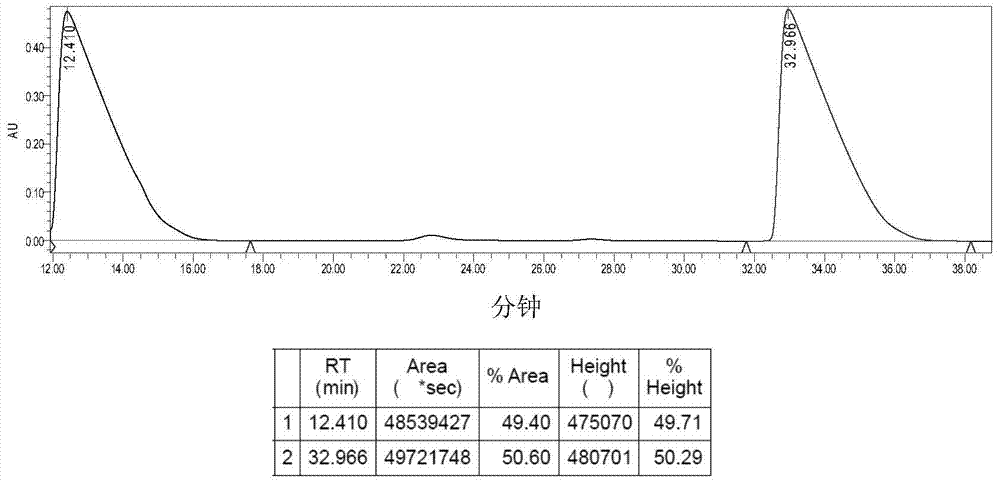
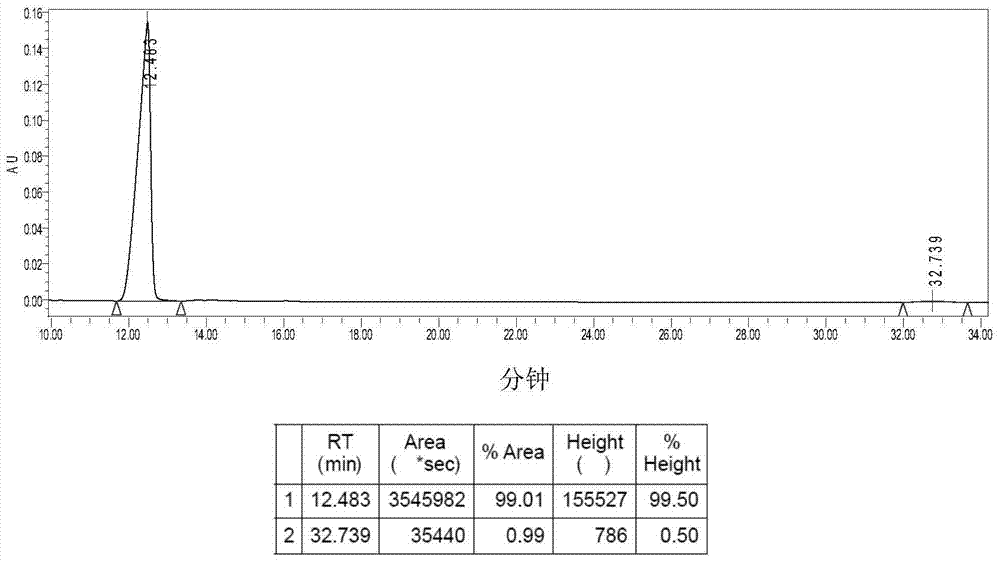
[0051] Suspend 0.41g (1.00mmol) of 1a in 120mL of deionized water, stir, add 0.30g (1.00mmol) of silver oxalate, react at 38°C in the dark for 24h, stop the reaction, and diatomaceous earth-assisted filtration to obtain a clear solution. The filtrate was concentrated to 10 mL, a large amount of solids precipitated out, refrigerated at 4°C, filtered, and dried in vacuo to obtain 0.30 g of 2a white solid with a yield of 71%. [α] D 30 =140.0° (c=0.20, DMF:H 2 O=1:1).Elem anal.Calcd for C 10 h 16 N 2 o 4 Pt: C, 28.37; H, 3.81; N, 6.62. Found: C, 28.63; H, 3.57; N, 6.83. IR (KBr, cm -1 ): 3274 (NH), 3131, 2943, 1704, 1660, 1589, 1372, 1153, 807; 1 H NMR (300MHz, d 6 -DMSO): δ1.17-1.65 (m, 8H, CH 2 of LR), 1.69-1.72 (m, 2H, CH of LR), 2.26(d, 2H, J=9.0Hz, 2×NH 2 CH ), 5.31-5.69 (m, 4H, 2×CH NH 2 ); 13 C NMR(d 6 -DMSO / TMS, ppm): δ19.22, 25.99, 31.17, 64.46, 165.87; ESI-MS: m / z [M+Na] + =446(100%).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap