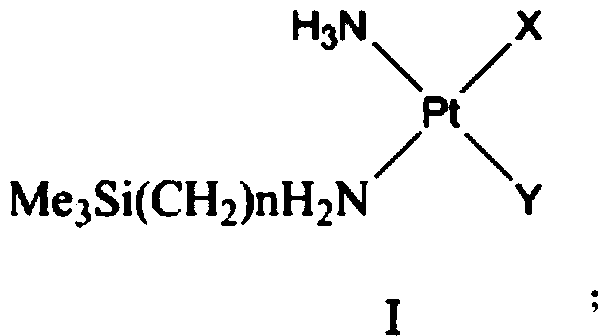
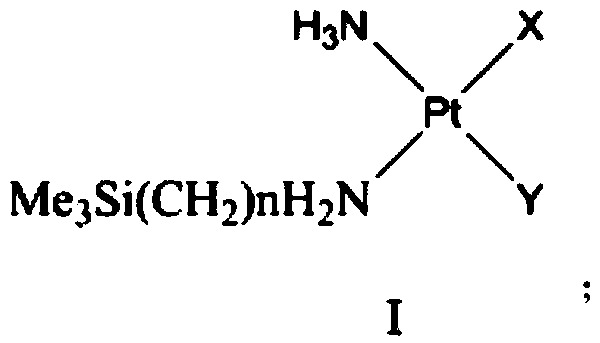
Monosilyl-containing platinum complex and application thereof
A technology of platinum complexes and monosilyl groups, which is applied in the field of platinum complexes containing monosilyl groups, can solve the problems of patients with kidney and nerve side effects, drug resistance, etc., and achieve reduction or elimination of repairing effects, elimination of repairing effects, and good curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Compound 2 Potassium Trichloro-Amine Platinate (II) and Compound 3 Cis-Aminotrimethylsilylethylaminochloroiodoplatinum
[0036]
[0037] (1) Raw materials (1) cis-diaminodichloroplatinum (5.0g, 0.017mol), tetraethylammonium (3.57 g, 1.2 times the equivalent) were dissolved in 130mL dimethylacetamide, and inert gas protection , Heated at 105°C for 12 hours, then distilled the solution to 50mL under reduced pressure, added 300mL of cyclohexane and ethyl acetate (1:1), and kept the suspension at -12°C for 12 hours. The precipitated crystals were filtered and dried in vacuo to give the crude product as an orange solid. Add 50 mL of deionized water, stir at 25°C for 30 minutes, and then filter. The obtained filtrate was put into Dowex 50W-X8 hydrogen ion exchange resin and stirred for 1 hour, then the filtrate and solid resin were separated by filtration, and the filtrate was concentrated to 5 mL. At 0°C, 2 mL of saturated potassium chloride solution was ...
Embodiment 2
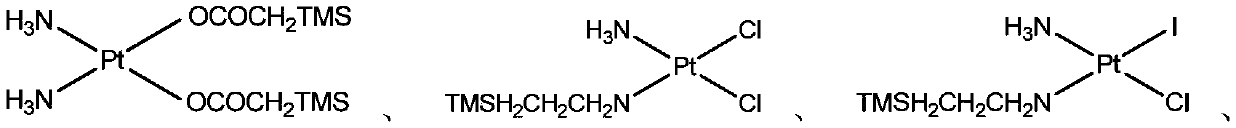
[0042] Preparation of compound 4 cis-aminotrimethylsilylethylaminodichloroplatinum
[0043]
[0044] (1) Compound 3 cis-aminotrimethylsilylethylaminochloroiodoplatinum (400 mg, 0.81 mmol) and silver nitrate (272 mg, 1.6 mmol) were dissolved in 4 mL of deionized water, and stirred at 22° C. in the dark for 6 hours. A small amount of activated charcoal was added and stirred for 30 minutes, then the solid was filtered. Then 1 mL of concentrated hydrochloric acid was added to the filtrate, and stirring was continued for 2 hours. The resulting yellow precipitate was filtered, the filter residue was rinsed with dimethyl ether, and vacuum-dried to obtain the pure product compound 4 cis-aminotrimethylsilylethylaminodichloroplatinum (315mg)
[0045] Compound 4 cis-aminotrimethylsilylethylaminodichloroplatinum: 195 Pt NMR (DMF-d 6 ): δ-2184 ppm; 1 HNMR (Aceton-d6 ):δ0.04ppm(s,9H,TMS),1.0(m,2H,CH 2 ),2.76(m,2H,CH 2 ),4.0(br,2H,NH 2 ),4.63(br,2H,NH 3 ).C 5 h 18 PtSiN 2 Cl 2...
Embodiment 3
[0047] Preparation of compound 5 cis-aminotrimethylsilylmethylaminochloroiodoplatinum
[0048]
[0049] At 0°C, dissolve 300 mg of the raw material compound 2 potassium trichloro-ammine platinum (II) in 4 mL of deionized water, add 240 mg of sodium iodide and 0.12 mL of 2-trimethylsilylethylamine, and keep this temperature for the reaction 4 hours. After the precipitate was filtered, the filter residue was washed with diethyl ether, and the filter residue was vacuum-dried to obtain a solid product compound 5 trimethylsilylmethylaminochloroiodoplatinum (290 mg).
[0050] Compound 5 Trimethylsilylaminochloroiodoplatinum: 195 Pt NMR (DMF-d 6 ): δ-2631ppm; 1 H NMR (DMF-d 6 ): δ0.11ppm(s,9H,TMS),2.36(m,2H,CH 2 ),4.2(br,2H,NH 2 ), 4.68(br,2H,NH 3 ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap