A kind of electrochemical sensor material for detecting urea and preparation method thereof
An electrochemical and sensor technology, which is applied in the field of material chemistry to achieve good electrocatalytic activity and promote electrochemical reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] To a 25 mL polytetrafluoroethylene liner was added potassium 5-nitroorotate monohydrate (0.5 mmol, 0.1286 g), 2,2'-bipyridine (0.5 mmol, 0.0781 g), and Co(Ac) 2 4H 2 O (0.5mmol, 0.1245g), add 15mL of distilled water, stir to obtain a reaction mixture; seal the polytetrafluoroethylene lining, put it in a stainless steel reaction kettle, put the stainless steel reaction kettle in an oven, and heat at 100°C for 72h , then naturally cooled to room temperature, opened the reaction kettle to obtain brownish-yellow block crystals; took out the crystals, and air-dried naturally to obtain the cobalt complex.
Embodiment 2
[0024] To a 25 mL polytetrafluoroethylene liner was added potassium 5-nitroorotate monohydrate (0.1 mmol, 0.0257 g), 2,2'-bipyridine (0.1 mmol, 0.0156 g), and Co(Ac) 2 4H 2 O (0.15mmol, 0.0374g), add distilled water 8mL, stir to obtain a reaction mixture; seal the polytetrafluoroethylene lining, put it in a stainless steel reaction kettle, put the stainless steel reaction kettle in an oven, and heat the reaction at 125°C for 48h , then naturally cooled to room temperature, opened the reaction kettle to obtain brownish-yellow block crystals; took out the crystals, and air-dried naturally to obtain the cobalt complex.
Embodiment 3
[0026] To a 25 mL polytetrafluoroethylene liner was added potassium 5-nitroorotate monohydrate (0.2 mmol, 0.0514 g), 2,2'-bipyridine (0.2 mmol, 0.0216 g), and Co(Ac) 2 4H 2 O (0.25mmol, 0.0623g), add distilled water 10mL, stir to obtain a reaction mixture; seal the polytetrafluoroethylene lining, put it in a stainless steel reaction kettle, put the stainless steel reaction kettle in an oven, and heat the reaction at 115°C for 60h , then naturally cooled to room temperature, opened the reaction kettle to obtain brownish-yellow block crystals; took out the crystals, and air-dried naturally to obtain the cobalt complex.
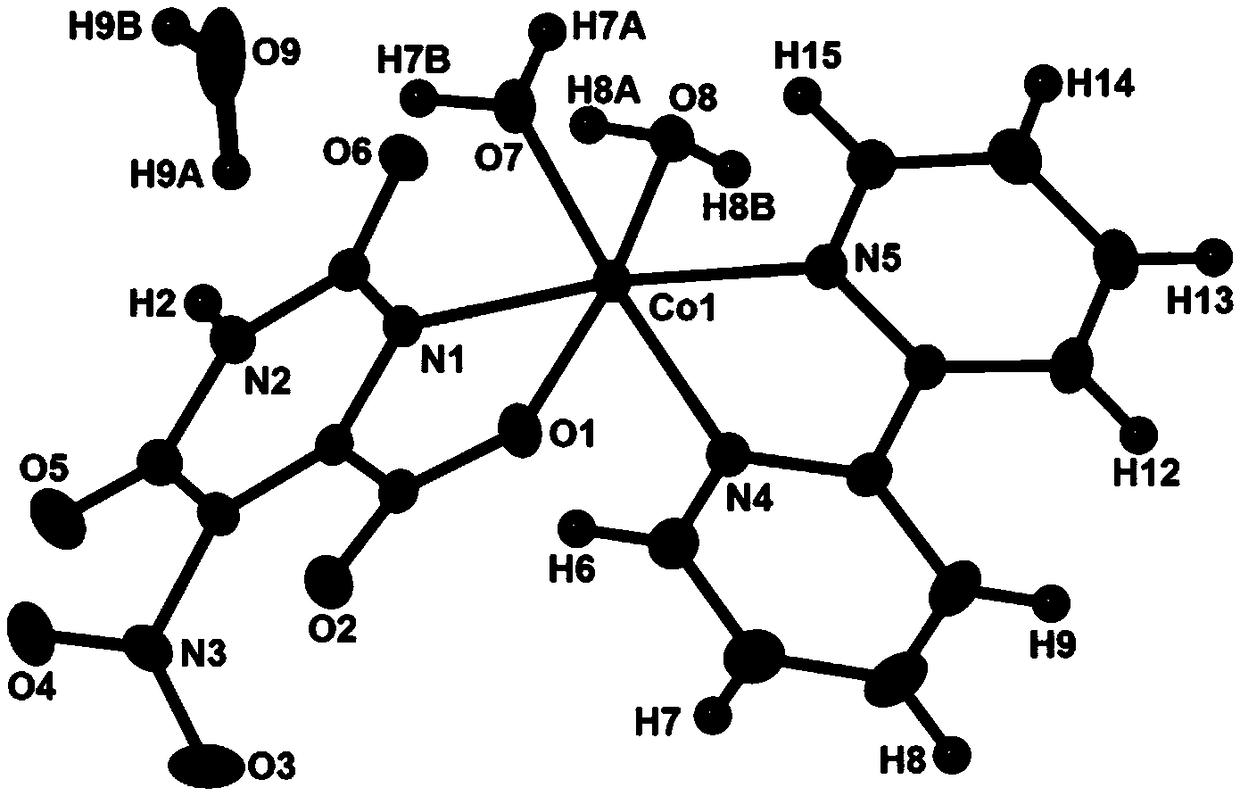
[0027] The brown-yellow crystals obtained in Examples 1-3 are subjected to structural testing with single crystal X-rays, and the results show that the brown-yellow crystals are cobalt coordination compounds, and its structural units are as follows: figure 1 As shown, its molecular formula is CoC 15 h 15 N 5 o 9 , the structural unit is [Co(C 5 HN 3 o 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com