Automatic issuance system of gene test report related to clopidogrel medication
A clopidogrel and gene detection technology, applied in the field of medical information management, can solve problems such as error-prone, weakened ability, time-consuming and labor-intensive, etc., and achieve the effects of reducing the occurrence rate of errors, saving time and cost, and being easy to use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
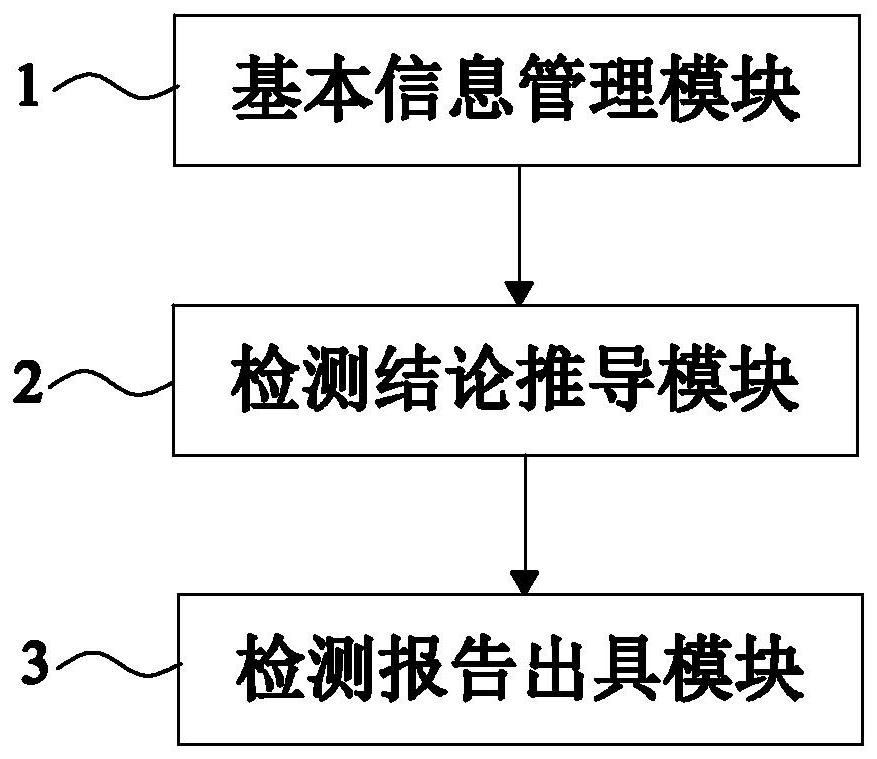
[0098] like figure 1 As shown, Embodiment 1 of the present invention provides a system for automatically issuing a gene detection report related to clopidogrel medication, including the following parts:
[0099] The basic information management module 1 is used to organize and store the clinical basic data of the patient and the detection results of clopidogrel medication related genes. The clopidogrel medication related genes include CYP2C19 gene, PON1 gene and ABCB1 gene. The CYP2C19 The genes include CYP2C19*2 locus, CYP2C19*3 locus and CYP2C19*17 locus, of which CYP2C19*2 locus, CYP2C19*3 locus and PON1 gene include GG, GA, AA three genotypes, CYP2C19*17 Both the locus and the ABCB1 gene include three genotypes: CC, CT, and TT;
[0100] The patient's clinical basic information can be manually entered or exported from the hospital's office system, including medical record number, name, gender, age, ethnicity, height, weight, department, bed number, sending physician, testi...
Embodiment 2
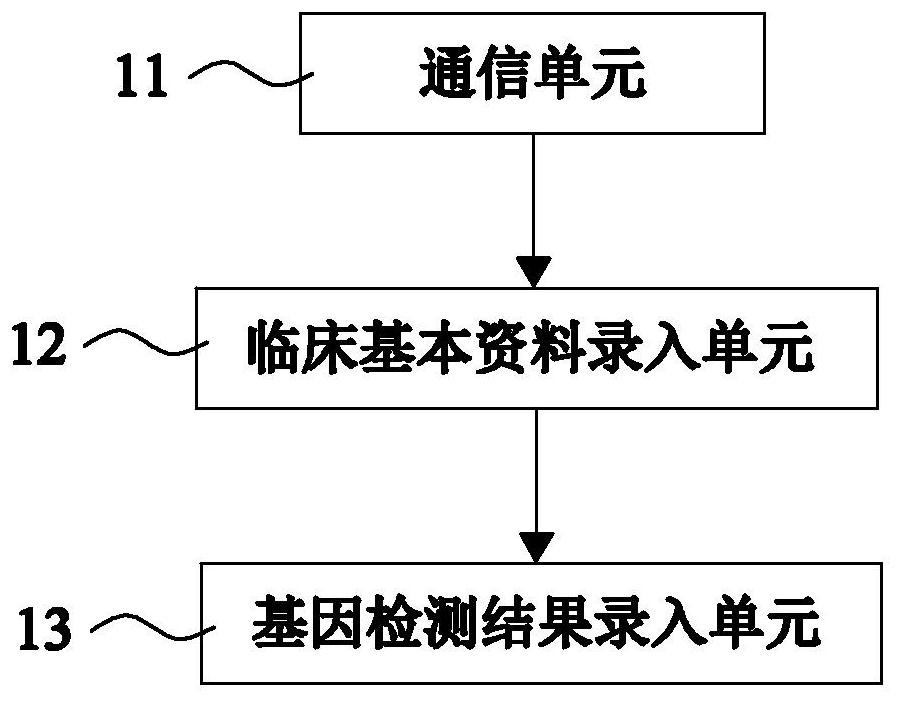
[0107] like figure 2 As shown, this embodiment 2 provides an automatic issuing system for clopidogrel medication-related gene detection reports on the basis of embodiment 1, and this embodiment 2 further defines that the basic information management module 1 includes the following parts:
[0108] The communication unit 11 is used to obtain the patient's case information and genetic testing information from the hospital information system;
[0109] The related systems of hospital registration information include HIS system (Hospital Information System, hospital information system), LIS system (Laboratory Information System, laboratory information system), etc. The relevant information of patients can be directly retrieved and copied from the above systems, so that in the No manual input is required when issuing the test report;
[0110] The clinical basic data entry unit 12 is used to extract and store the clinical basic information from the case information;
[0111] The ge...
Embodiment 3
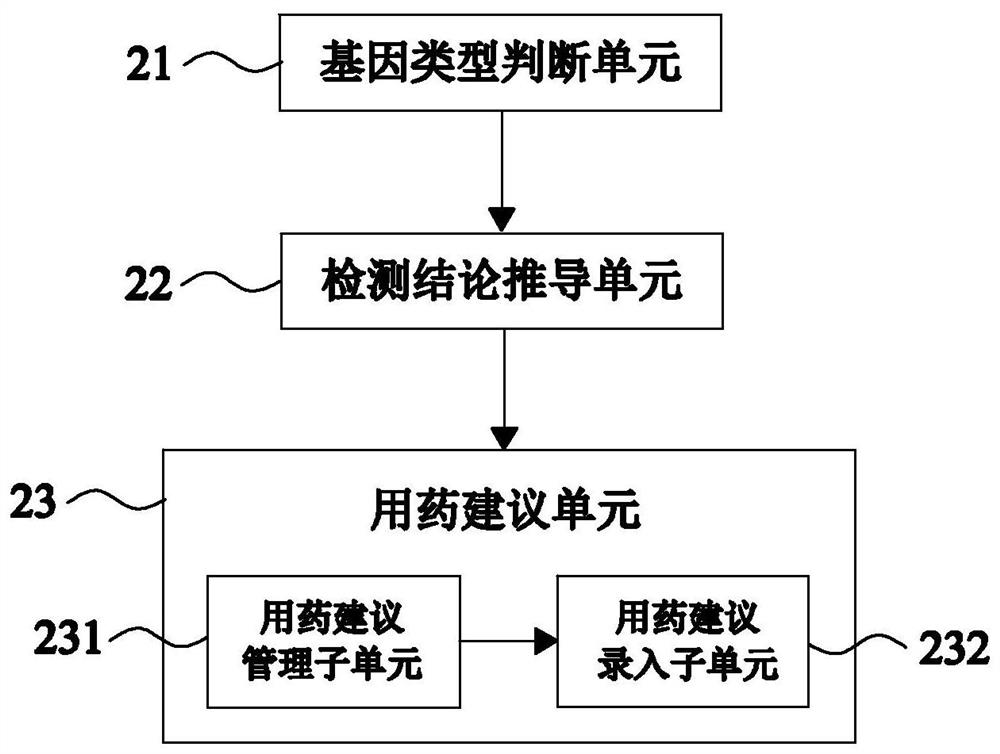
[0115] like image 3 As shown, this embodiment 3 provides an automatic issuing system for clopidogrel medication-related gene detection reports on the basis of embodiment 1, and this embodiment 3 further defines that the detection conclusion derivation module 2 includes the following parts:
[0116] The genotype judgment unit 21 is configured to judge the genotypes of the CYP2C19 gene, the PON1 gene and the ABCB1 gene of the patient according to the detection results of the clopidogrel medication-related genes;
[0117] CYP2C19 enzyme is closely related to the resistance reaction after clopidogrel administration; PON1 enzyme also plays a key role in the process of converting clopidogrel into active products, but its role is not as prominent as CYP2C19 enzyme; It plays a role in intestinal absorption into the blood, but its role is weaker than that of the PON1 enzyme. Therefore, it is necessary to judge the genotypes of the above three genes separately, so that the detection c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com