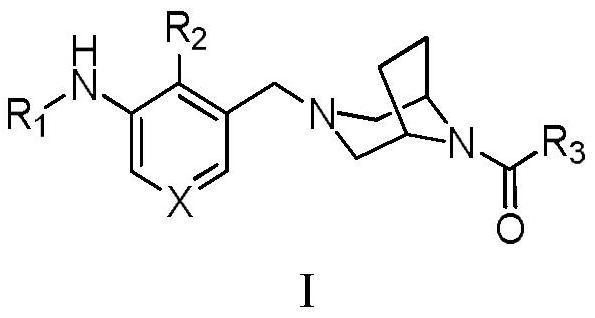
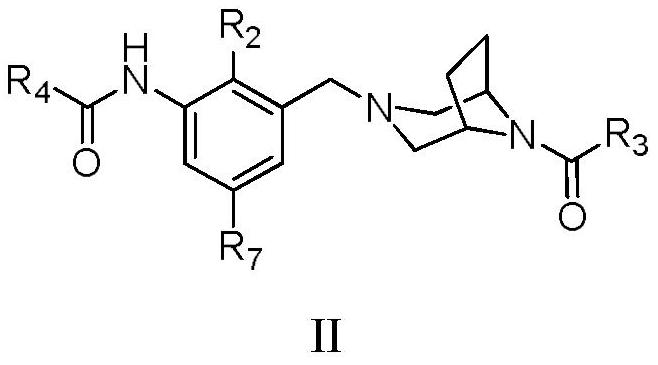
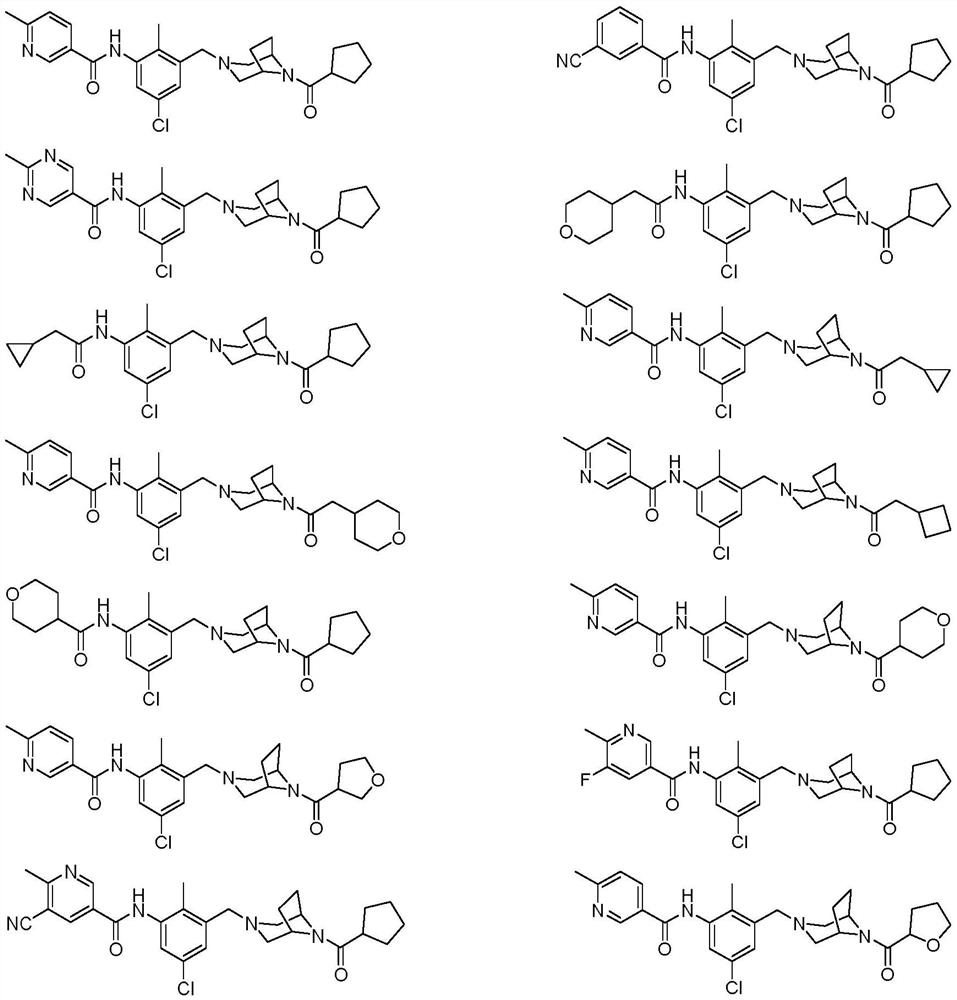
Bridged ring piperazine derivatives or salts thereof, preparation method and use thereof
A derivative, piperazine technology, applied in the field of chemical medicine, can solve the problem of reduced ability of strain differentiation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: N-(5-chloro-3-((8-(cyclopentanecarbonyl)-3,8-diazabicyclo[3.2.1]oct-3-yl)methyl Base)-2-methoxyphenyl)-6-methylnicotinamide
[0076] N-(5-chloro-3-((8-(cyclopentanecarbonyl)-3,8-diazabicyclo[3.2.1]octan-3-yl)methyl)-2-methylphenyl)-6-methylnicotinamide
[0077]
[0078] Step 1: Preparation of 5-chloro-2-methyl-3-nitrobenzoic acid
[0079] At 0°C, 5-chloro-2-methylbenzoic acid (10g, 58.8mmol) was dissolved in concentrated sulfuric acid (98%, 150mL), the mixture was stirred at 0°C for 10 minutes, until the solid was dissolved, nitric acid (65%, 20mL) It was added dropwise into the solution, and after the dropwise addition was completed, the mixture was naturally warmed to room temperature and stirred for 5 hours. The reaction solution was poured into an ice-water mixture (500 mL), filtered, and the filter cake was washed with water (100 mL) to obtain 12.0 g of a light yellow solid with a yield of 95.2%. 1 H-NMR (400MHz, CDCl 3 )δ: 8.17 (s, 1H), 8.01 (s, ...
Embodiment 2
[0094] Example 2: N-(5-chloro-3-((8-(cyclopentanecarbonyl)-3,8-diazabicyclo[3.2.1]oct-3-yl)methyl Base)-2-methylphenyl)-3-aminobenzamide
[0095] N-(5-chloro-3-((8-(cyclopentanecarbonyl)-3,8-diazabicyclo[3.2.1]octan-3-yl)methyl)-2-methylphenyl)-3-cyanobenzamide
[0096]
[0097] At 0°C, to a dichloromethane solution (5 mL) containing 6-methylnicotinic acid (88.2 mg, 0.6 mmol), 2 drops of a catalytic amount of DMF, and oxalyl chloride (152.4 mg, 1.2 mmol) were added dropwise. dichloromethane solution. After the dropwise addition was completed, it was raised to room temperature to react for 3 hours, and the solvent was evaporated to dryness under reduced pressure; at 0°C, the residue was dissolved in dichloromethane, and the solution containing (3-(3-amino-5-chloro-2-methyl Benzyl)-3,8-diazabicyclo[3.2.1]oct-8-yl)(cyclopentyl)methanone (150mg, 0.4mmol) and dichlorotriethylamine (121.2mg, 1.2mmol) In methane solution (5 mL), the dropwise addition was completed, raised to ...
Embodiment 3
[0098] Example 3: N-(5-chloro-3-((8-(cyclopentanecarbonyl)-3,8-diazabicyclo[3.2.1]oct-3-yl)methyl Base)-2-methoxyphenyl)-2-methylpyrimidine-5-carboxamide
[0099] N-(5-chloro-3-((8-(cyclopentanecarbonyl)-3,8-diazabicyclo[3.2.1]octan-3-yl)methyl)-2-methylphenyl)-2-methylpyrimidine-5-carboxamide
[0100]
[0101] At 0°C, in a dichloromethane solution (5 mL) containing 2-methyl-5-pyrimidinecarboxylic acid (82.8 mg, 0.6 mmol), 2 drops of a catalytic amount of DMF, and oxalyl chloride (152.4 mg, 1.2 mmol) in dichloromethane solution; after the dropwise addition, rise to room temperature and react for 3 hours, evaporate the solvent to dryness under reduced pressure; at 0°C, after dissolving the residue with dichloromethane, add dropwise 5-Chloro-2-methylbenzyl)-3,8-diazabicyclo[3.2.1]oct-8-yl)(cyclopentyl)methanone (150mg, 0.4mmol) and triethylamine (121.2 mg, 1.2mmol) in dichloromethane solution (5mL), the dropwise addition was completed, and it was raised to room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com