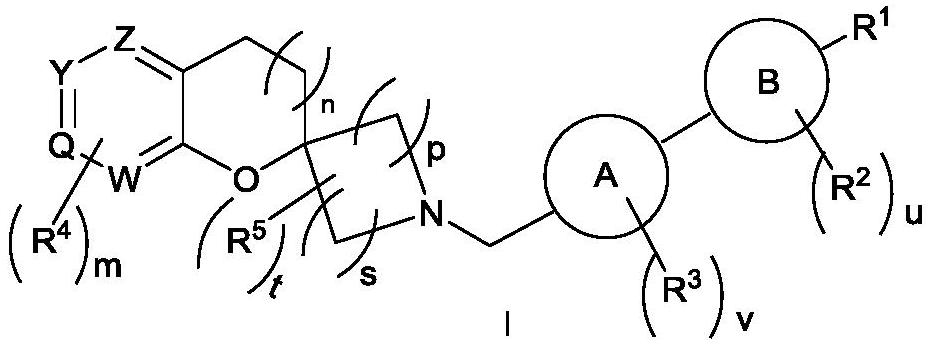
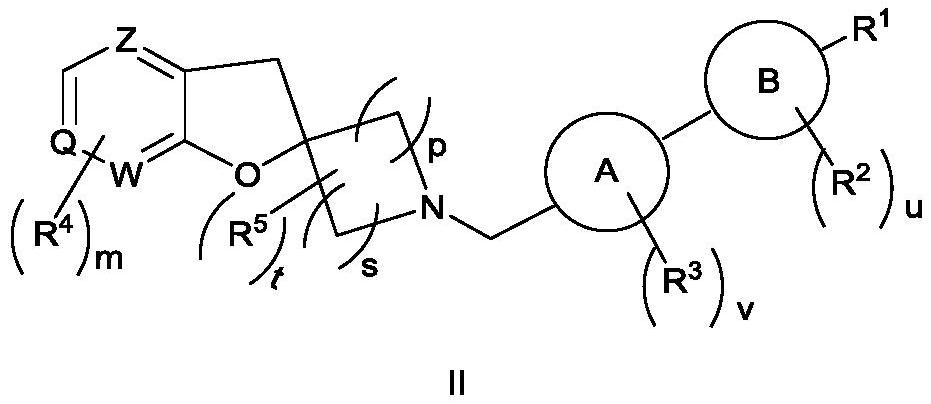
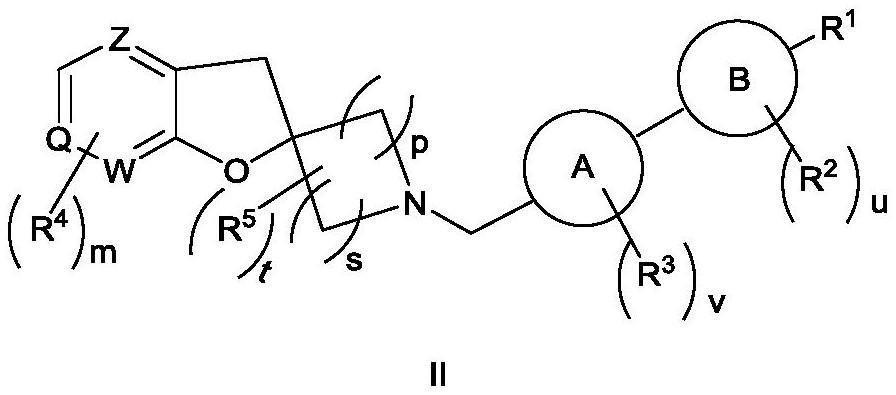
Spiroheterocyclic compound as well as preparation method and application thereof
A spiro-heterocycle, compound technology, applied in the direction of drug combination, organic chemistry, digestive system, etc., to achieve the effect of inhibiting production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0351] Preparation example 1 (preparation of key intermediate)
[0352]
[0353] first step:
[0354] Compound 1-a (20g, 85.822mmol, 1.00equiv) and trifluoromethyltrimethylsilane (TMSCF 3 , 61.02g, 429.111mmol, 5.00equiv) in tetrahydrofuran (200mL) was added tetrabutylammonium fluoride (54.15g, 171.644mmol, 2.00equiv) and stirred overnight at room temperature. After the reaction was monitored by LCMS until the reaction was complete, it was quenched by adding 1N HCl at 0°C. The reaction solution was extracted with ethyl acetate, anhydrous Na 2 SO 4 (sodium sulfate) dry. The reaction solution was concentrated and separated by column chromatography (EA / PE, 15%-30%) to obtain light yellow oily compound 1-b (11.80 g, 34.602 mmol, 40.32% yield). LCMS:(ESI,m / z):[M-1] - = 338.75. 1 H NMR (300MHz, chloroform-d) δ7.66 (dd, J = 8.6, 7.0Hz, 1H), 7.52 (dd, J = 9.6, 2.2Hz, 1H), 7.45–7.33 (m, 1H), 3.56 ( s, 1H).
[0355] Step two:
[0356] Add 4-(4,4,5,5-tetramethyl- 1,3,2-Diox...
preparation example 2
[0357] Preparation example 2 (preparation of key intermediate)
[0358]
[0359] first step:
[0360] At 0°C, 2 mL of sodium nitrite (NaNO 2 ) (293 mg, 4.24 mmol, 1.1 equiv) in water, followed by the dropwise addition of HCl (6M, 2 mL, 11.58 mmol, 3.0 equiv) at 0°C. The reaction mixture was stirred for an additional 1 hour at 0°C. Additional KI (673mg, 4.05mmol, 1.05eq) was added at 0°C and then stirred overnight at room temperature. The completion of the reaction was monitored by LCMS, and water (30 mL) was added to dilute the reaction, and extracted with ethyl acetate (3×15 mL). The organic phases were combined, washed with saturated brine and concentrated to dryness, and the residue was separated by column chromatography (EA / PE, 10%-50%) to obtain compound 2-b (912mg, 2.46mmol, 63.87% yield), yellow oil liquid. LCMS:(ESI,m / z):[M-1] - = 368.9.
[0361] Step two:
[0362] To compound 2-b (23.00g, 62.16mmol, 1equiv) in 1,4-dioxane solution (300mL), add diboronic aci...
preparation example 3
[0363] Preparation example 3 (preparation of key intermediate)
[0364]
[0365] (4-formylphenyl)boronic acid (2.43g, 16.215mmol, 1.2 eqiv), tetrakistriphenylphosphine palladium (1.56 g, 1.351 mmol, 0.10 eqiv) and potassium carbonate (5.6 g, 40.537 mmol, 3.00 eqiv). The reaction mixture was stirred overnight at 80 °C under nitrogen protection. The completion of the reaction was monitored by LCMS, the solvent was removed in vacuo, and the residue was separated by column chromatography (EA / PE, 0%-40%) to obtain a crude product, which was purified by n-hexane to obtain compound 3 (2.491g, 7.153mmol, 52.94% yield rate), a yellow solid. LCMS:(ESI,m / z):[M-1] - =347.00. 1 H NMR (400MHz, DMSO-d 6 )δ10.07(s,1H),8.84(s,1H),8.02(d,J=8.3Hz,2H),7.99–7.88(m,4H),7.82(d,J=8.3Hz,2H). 19 F NMR (377MHz, DMSO-d 6 ) δ-73.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com