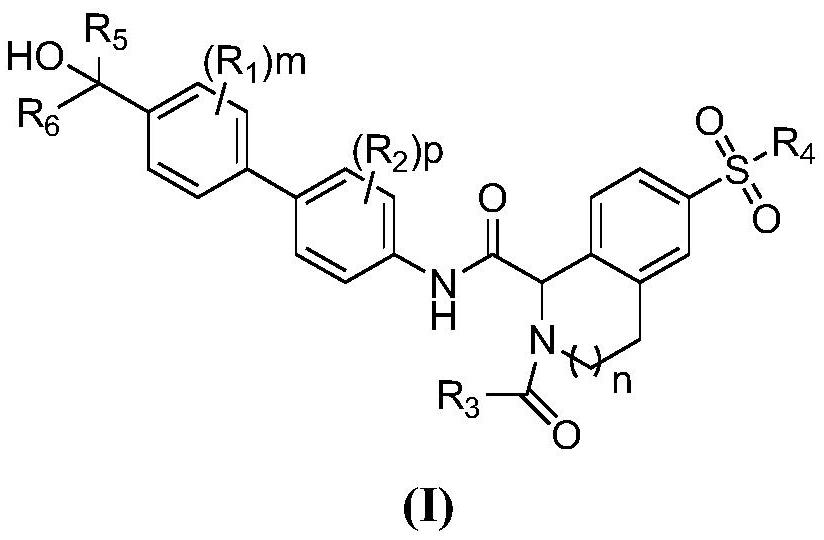
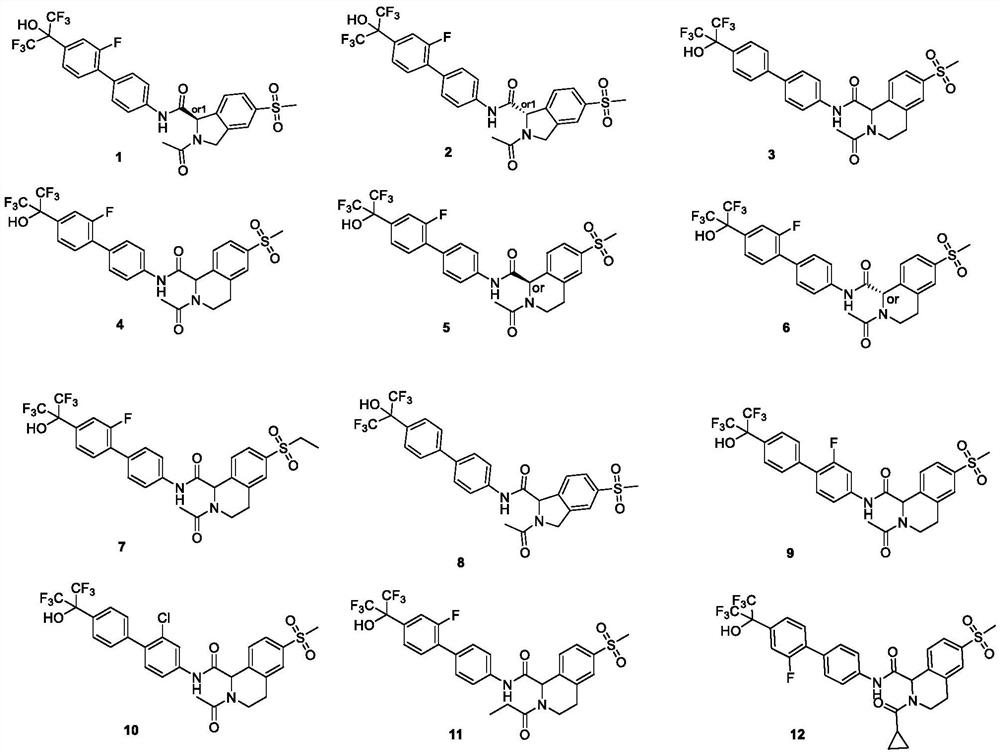
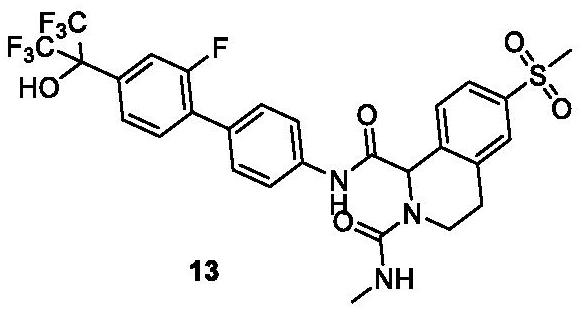
Biaryl compound useful as ROR gamma modulator
A compound, solvate technology, applied in the field of chemical medicine, can solve the problem of reduced ability of differentiation and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0217] The technical solutions of the present invention are further described below, but the scope of the invention is not limited to these embodiments. Alternatively, alterations that do not depart from the concepts of the present invention are included within the scope of the invention.
[0218] The experimental method of the specific condition is not indicated in the following examples, usually in accordance with the conventional conditions of this type, or according to the conditions recommended in the manufacturer. Unless otherwise stated, the number of percentages and copies is the number of weights and weights. The ratio of liquid is a volume ratio unless otherwise stated.
[0219]Materials and reagents used in the following embodiments unless otherwise specified and can be obtained from commercial sources, or prepared according to the present application have disclosed a method similar to the method according to the prior art.
[0220] As used herein, the chemical name IUP...
Embodiment 1 and Embodiment 2
[0243] Example 1 and Example 2: R-2- acetyl -N- (2'- fluoro-4 '- (1,1,1,3,3,3-hexafluoro-2-hydroxy-2-yl ) - [1,1'-biphenyl] -4-yl) -5- (methylsulfonyl) isoindoline-1-carboxamide or S-2- acetyl -N- (2'- fluoro - 4 '- (1,1,1,3,3,3-hexafluoro-2-hydroxy-2-yl) - [1,1'-biphenyl] -4-yl) -5- (methyl sulfonamide acyl) isoindoline-1-carboxamide
[0244]
[0245] Synthesis of 1,1,1,3,3,3-hexafluoro-propan-2-ol - Intermediate 2- ([1,1'-biphenyl] -4-amino-2-fluoro-4'-yl)
[0246] Step 1: 4-bromo-perfluorophenyl Synthesis of 3-fluoro-benzoate
[0247]
[0248] Sequentially bromo-3-fluorobenzoic acid (30.0g, 137mmol), pentafluorophenol (28.2g, 153mmol) and dicyclohexyl carbodiimide (31.9g, 155mmol) was dissolved in dry tetrahydrofuran (900 mL) in . The reaction mixture was stirred at room temperature overnight, complete reaction was monitored by TLC. The reaction mixture was filtered, the filtrate was concentrated under reduced pressure to give the crude product Purification by silica gel co...
Embodiment 3
[0293] Example 3: 2-Acetyl-N- (4 '- (1,1,1,3,3,3-hexafluoro-2-hydroxypropane-2-yl) - [1,1'-biphenyl] -4-yl) -6- (methylsulfonyl) -1,2,3,4-tetrahydroisoquinoline-1-formamide
[0294]
[0295] Synthesis of intermediate 2- (tert-butoxycarbonyl) -6- (methylsulfonyl) -1,2,3,4-tetrahydroisoquinoline-1-formic acid
[0296] Step 1: 3- (2-aminoethyl) phenolic bromide synthesis
[0297]
[0298] 2- (3-methoxyphenyl) ethylamine (9.00 g, 59.5 mmol) was dissolved in a 40% hydrobromide solution (120 g, 595 mmol). The reaction mixture was stirred at 110 ° C for 6 hours, and the LC-MS monitored reaction was complete. The reaction mixture was concentrated under reduced pressure to give the target compound (9.00 g, crude, light brown solid). LC-MS (ESI) M / Z: 179.1 [M + ACN + H] + .
[0299] Step 2: Synthesis of 6-hydroxy-1,2,3,4-tetrahydroisoquinoline-1-formate hydrochloride
[0300]
[0301] 3- (2-aminoethyl) phenolic bromide (9.00 g, 65.6 mmol) was dissolved in ethanol (90 mL). 50% acetan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com