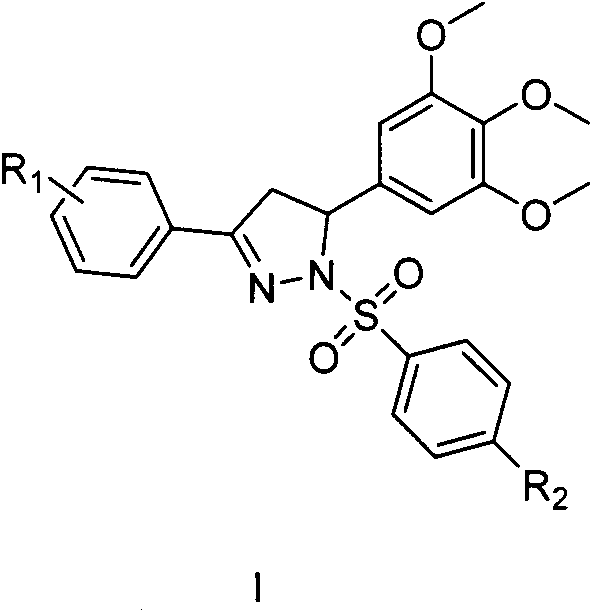
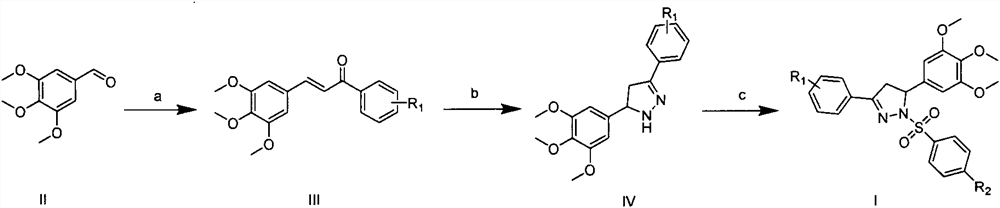
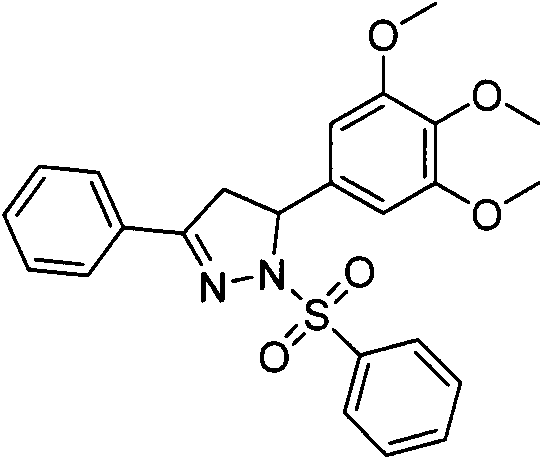
Preparation and application of a class of benzenesulfonyldihydropyrazole derivatives
A technology of benzenesulfonyldihydropyrazole and its derivatives, which is applied in the fields of medical preparations containing active ingredients, drug combinations, organic chemistry, etc., can solve the problems of large toxic and side effects, difficult synthesis, unstable structure, etc., and achieve The effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 3-phenyl-1-benzenesulfonyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydropyrazole (I-1)
[0020]
[0021] Under ice-bath conditions, add 3,4,5-trimethoxybenzaldehyde (5.0mmol) and acetophenone (5mmol) into absolute ethanol (20mL), and add 10% NaOH aqueous solution dropwise to the reaction solution while stirring (1mL), TLC tracking monitoring, complete reaction, the reaction solution was poured into saturated brine, a yellow solid precipitated, filtered and dried to obtain compound III-1. Under reflux conditions, compound III-1 (1 mmol) and 80% hydrazine hydrate (1 mL) were added to isopropanol (3 mL), and TLC was monitored. After complete reaction, the reaction solution was placed in cold hydrazine at -20°C and stirred until A white solid precipitated out, and compound IV-1 was obtained by suction filtration, which was quickly used for the next reaction. Under ice bath conditions, compound IV-1 (1 mmol), anhydrous potassium carbonate (1 mmol) and benzenesulf...
Embodiment 2
[0023] Preparation of 1-(4-chlorobenzenesulfonyl)-3-phenyl-5-(3,4,5-trimethoxyphenyl)-4.5-dihydropyrazole (I-2)
[0024]
[0025] The preparation method refers to Example 1. A white powder was obtained, yield: 77.1%, m.p.206-207°C. 1 H NMR (600MHz, DMSO) δ7.86 (d, J=8.7Hz, 2H), 7.74-7.66 (m, 4H), 7.51-7.42 (m, 3H), 6.69 (s, 2H), 4.94 (dd, J=11.0, 9.6Hz, 1H), 3.76(s, 6H), 3.72-3.65(m, 4H), 3.25(dd, J=17.7, 9.4Hz, 1H).MS(ESI): 487.97[M+H ] + ;Anal.Calcd.for C 24 h 23 ClN 2 o 5 S: C, 59.20; H, 4.76; N, 5.75. Found: C, 59.17; H, 4.78; N, 5.77.
Embodiment 3
[0027] Preparation of 3-phenyl-1-p-methylbenzenesulfonyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydropyrazole (I-3)
[0028]
[0029] The preparation method refers to Example 1. A white powder was obtained, yield: 77.2%, m.p.244-245°C. 1 H NMR (600MHz, DMSO) δ7.75 (d, J=8.3Hz, 2H), 7.69 (dd, J=8.0, 1.4Hz, 2H), 7.45 (ddd, J=24.6, 12.0, 6.9Hz, 5H) , 6.71(s, 2H), 4.92-4.82(m, 1H), 3.77(s, 6H), 3.67(s, 3H), 3.62(dd, J=17.7, 11.3Hz, 1H), 3.20(dd, J =17.7, 9.8Hz, 1H), 2.37(s, 3H).MS(ESI): 467.55[M+H] + ;Anal.Calcd.for C 25 h 26 N 2 o 5 S: C, 64.36; H, 5.62; N, 6.00. Found: C, 64.34; H, 5.60; N, 6.02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com