a kit
A kit and catheter ablation technology, applied in the medical field, can solve the problem that there is no sensitive and specific biomarker for assessing the late recurrence risk of atrial fibrillation catheter ablation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Clinical research on late recurrence and plasma TMAO after catheter ablation of atrial fibrillation
[0025] 1. Case selection
[0026] A total of 60 patients with atrial fibrillation who were about to undergo catheter ablation for the first time were selected, and the recurrence of atrial fibrillation was followed up in March, June, September, and December respectively, and they were divided into non-late recurrence group and postoperative recurrence group ; 30 patients with paroxysmal supraventricular tachycardia were selected as the control group. [Exclusion criteria: previous history of atrial fibrillation ablation; presence of left atrial thrombus; presence of anticoagulation contraindications; history of myocardial infarction within six months; congestive heart failure; severe chronic organic diseases; hyperthyroidism; pregnancy; mental illness abnormal】
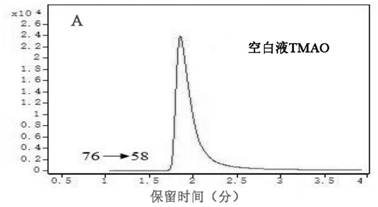
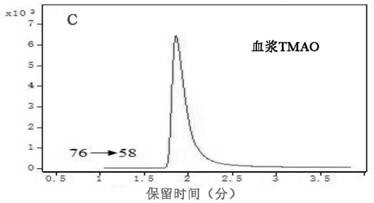
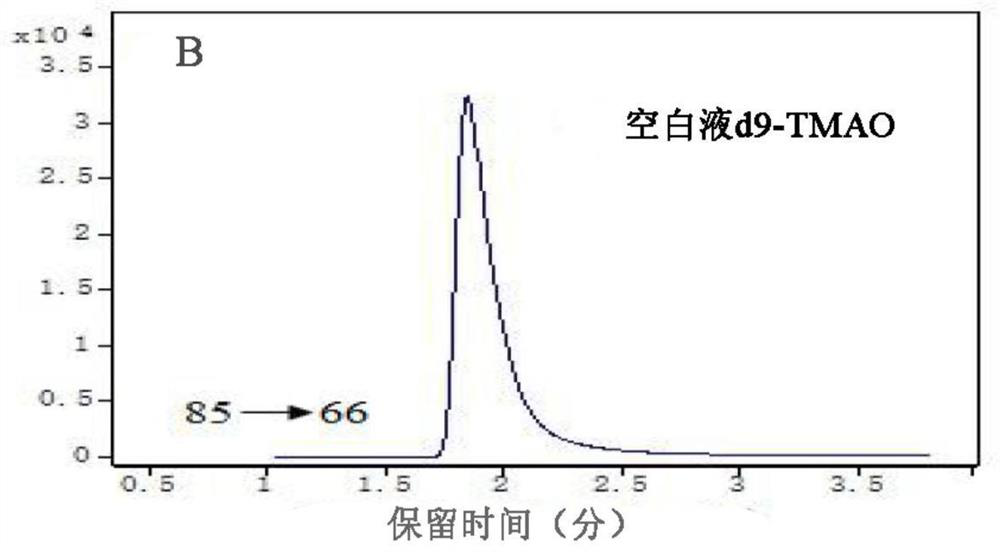
[0027] 2. Determination results of plasma TMAO
[0028] In the morning before the operation, 3m...
Embodiment 2
[0037] Embodiment 2: the specific case in embodiment 1
[0038] patient one
[0039] 1. Clinical data
[0040] The patient, male, 53 years old, was admitted to the hospital due to "palpitations for more than half a year". Diagnosis: atrial fibrillation (paroxysmal), underwent circular pulmonary vein isolation, intraoperative matrix mapping, no left atrial low voltage area was found. Sinus rhythm was restored postoperatively. Reexamination at 3 months, 6 months, 9 months, and 12 months after the operation showed no recurrence of atrial fibrillation.
[0041] Second, the determination of plasma TMAO:
[0042] TMAO high-performance liquid chromatography tandem mass spectrometry kit assay method: (1) Collect 3ml of fasting venous blood from the patient before operation, put it in an EDTA anticoagulant vacuum tube, centrifuge at 3500r for 10min at 4°C, separate the plasma, and store it in a -80°C refrigerator. (2) Take out the kit and configure standard products: mother solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com