Checkpoint regulator antagonists
An antibody and antigen technology, applied in the field of bispecific and trispecific checkpoint regulator antagonists, checkpoint regulator antagonists, can solve the problem that the host cannot eliminate cancer cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0347] Example 1: Production of Monoclonal Antibodies
[0348] Monoclonal antibodies (mAbs) of the present application are generated and screened using techniques well known in the art, see eg Harlow and Lane (1988) Antibodies, A Laboratory Manual, (Antibodies, A Laboratory Manual) Cold Spring Harbor Publishing, New York. Antigen-specific hybridoma monoclonal antibodies are cloned, sequenced and engineered using techniques well known in the art, see e.g. Lo.B.K.C Methods in Molecular Biology TM , Volume 248 2004 Antibody Engineering (Methods in Molecular Biology TM . Volume 248 2004. Antibody Engineering).
Embodiment 2
[0349] Example 2: TIGIT Binder Screening Assay
[0350] Coat 1 μg / ml TIGIT-His protein overnight at 4°C and block with 1% BSA in PBS for 1 hour at room temperature, then incubate with 50 μl hybridoma supernatant for 1 hour at room temperature, pass through anti-mouse IgG HRP Detect mouse IgG for 30 minutes, then add TMB substrate and pass through the addition of 2N H 2 SO 4 Stop the reaction. Wells with an OD of at least 5 times background were selected as positive binders.
Embodiment 3
[0351] Example 3: TIGIT Blocker Screening Assay
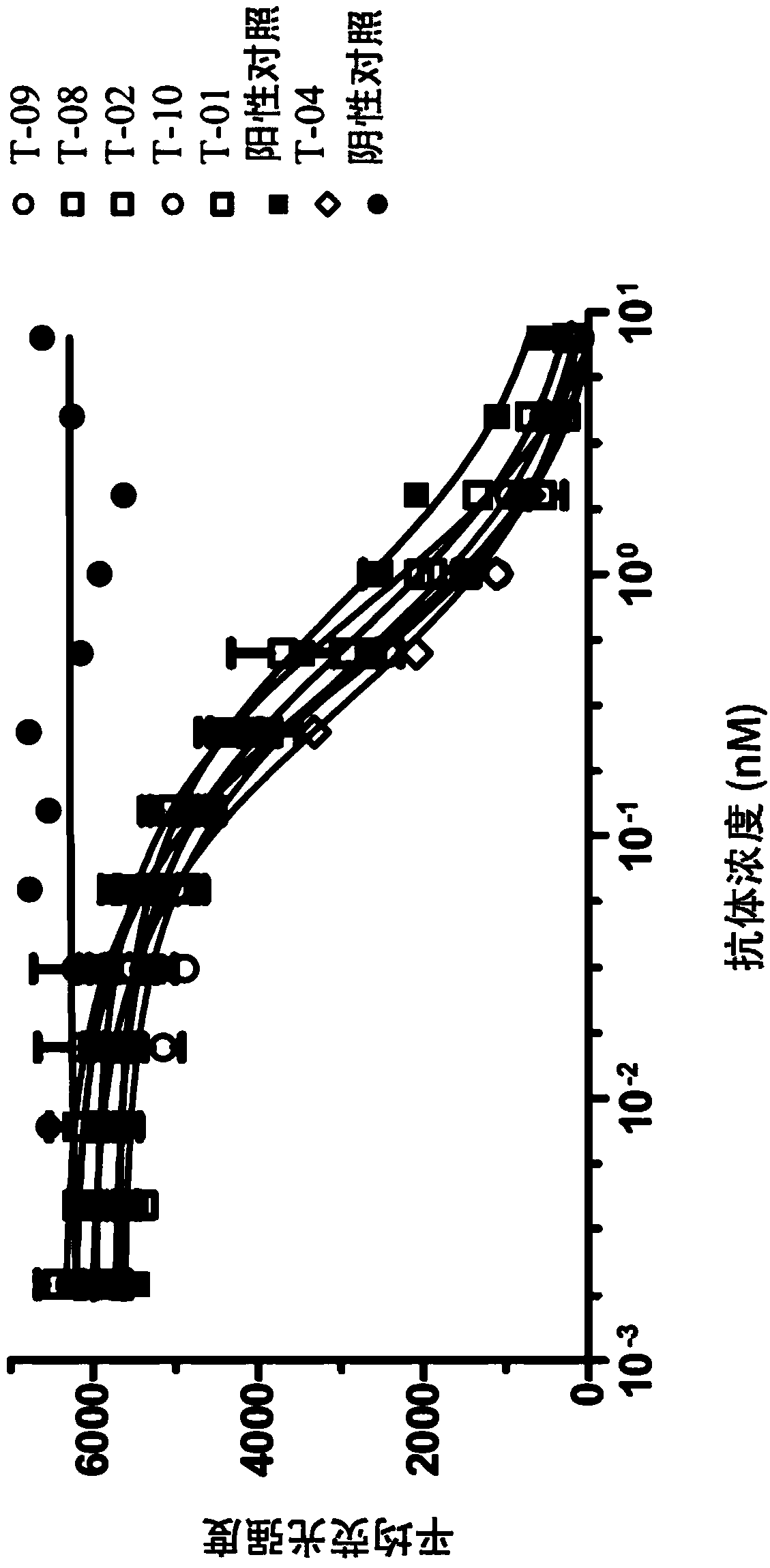
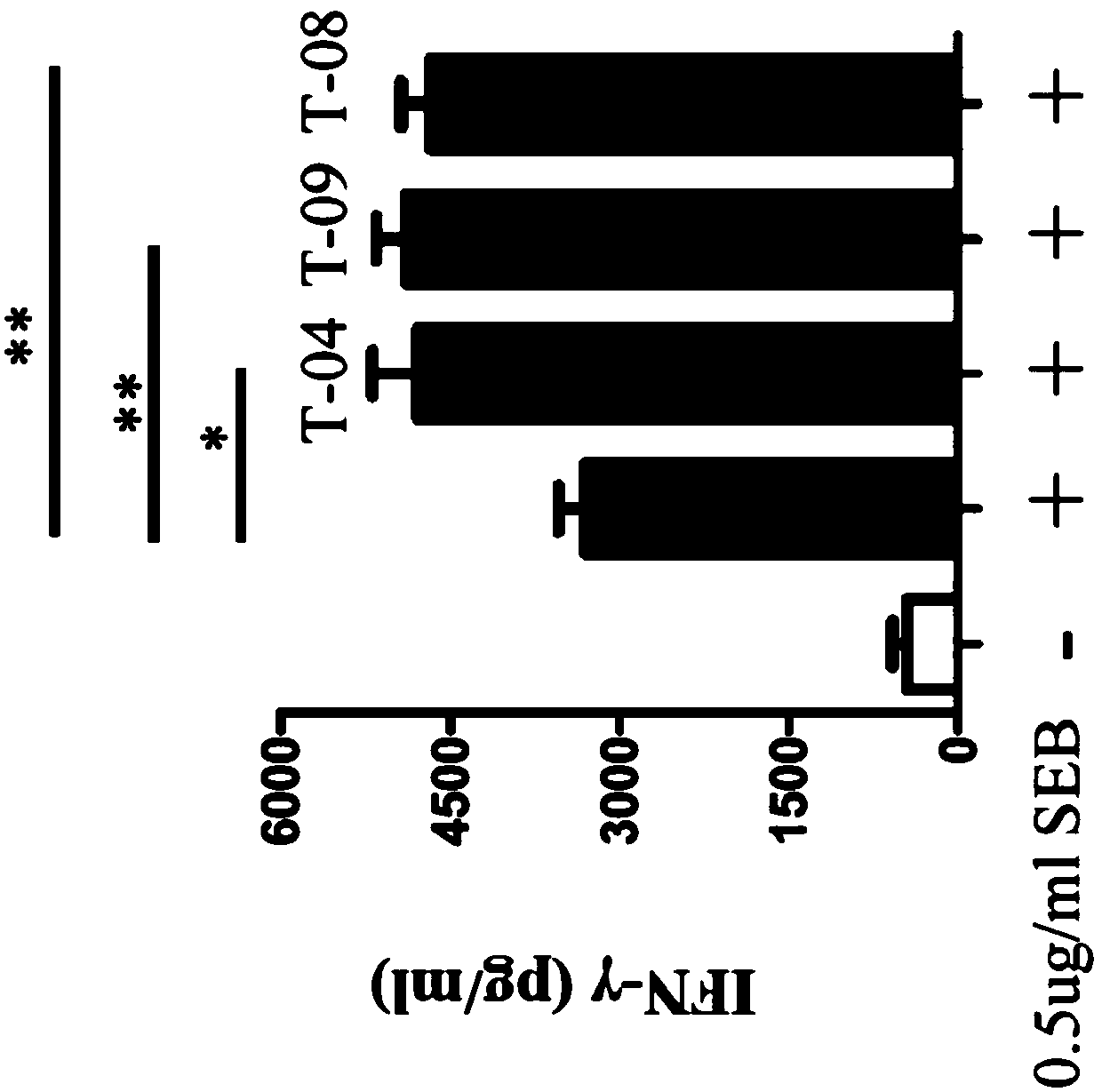
[0352] 3 x 10 at 4°C 4TIGIT+CHO-K1 cells were incubated in 50 μl hybridoma supernatant for 20 minutes, and then human PVR human Fc tag fusion protein was added to a final concentration of 0.6 μg / ml; after incubation at 4°C for 30 minutes, the cells were incubated with FACS buffer ( 0.5% BSA, 2mM EMTA in PBS) to wash the cells, and then incubate the cells with 1 μg / ml PE-labeled anti-human Fc antibody at 4°C for 20 minutes. Cells were then washed with FACS buffer, then resuspended in 7-amino-actinomycin D (7AAD) solution, and analyzed with the iQue intellicyt system. Wells that completely blocked TIGIT and PVR (Fc tag) binding were selected as blocking agents. In the first screen, 18 blockers were identified from 65 binders. In the second screen, 17 blockers were identified from 30 binders. figure 1 Exemplary results of competition assays of various mouse anti-TIGIT monoclonal antibodies are shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com