Novel glutaminyl cyclase inhibitors and the use thereof in treatment of various diseases
A technology of glutaminyl cyclase and use, which is applied in the field of pharmacology and medicine, and the chemistry of organic compounds, which can solve the problems of immune system cell side effects and reduce the metabolic stability of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
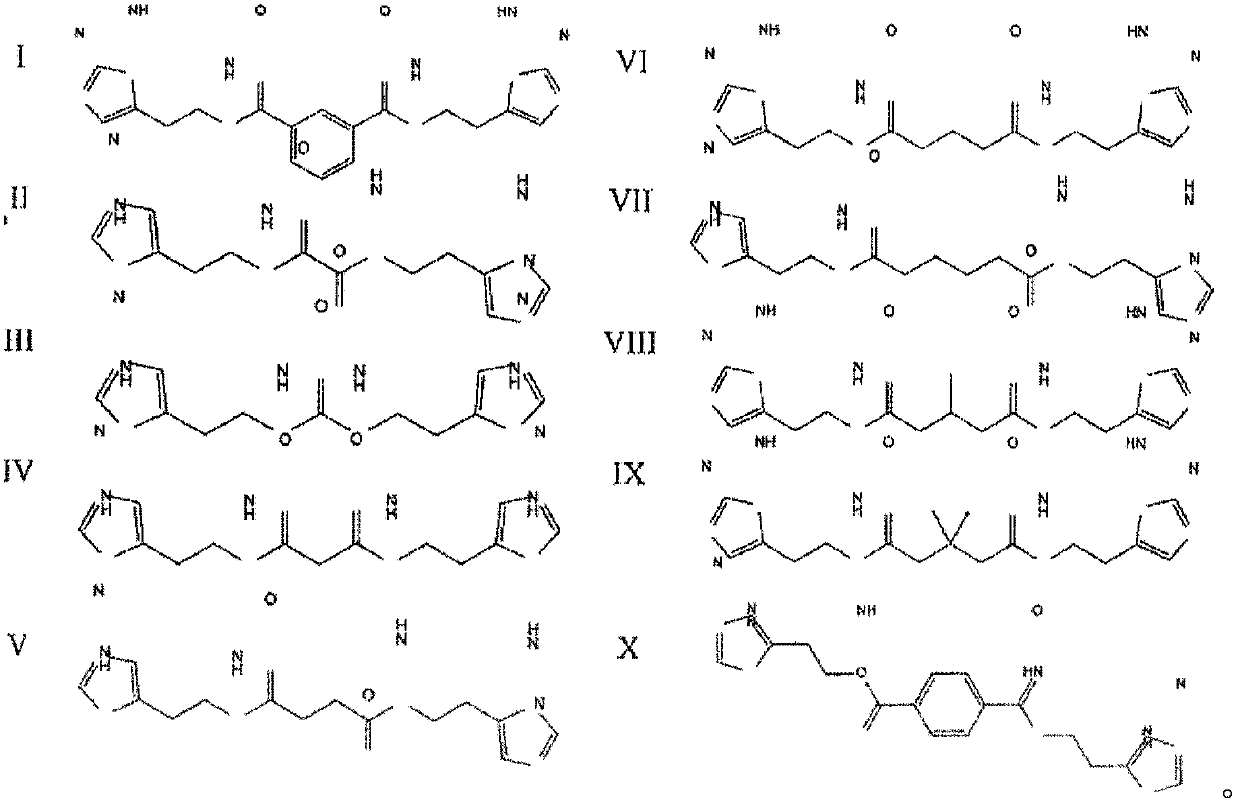
[0138] Obtaining of compounds according to the invention
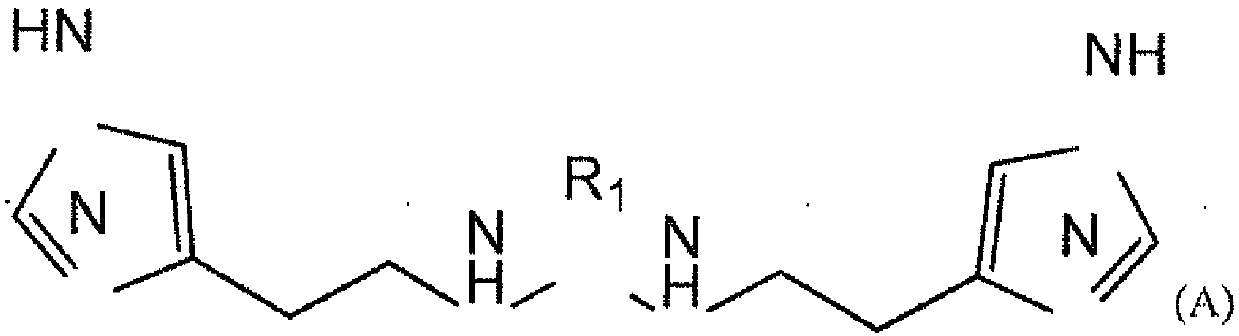
[0139] Methods for the production of compounds I to X are disclosed in the invention application RU2013 / 116822. The ability of similar compounds to complex or chelate metal ions is described in the same application.
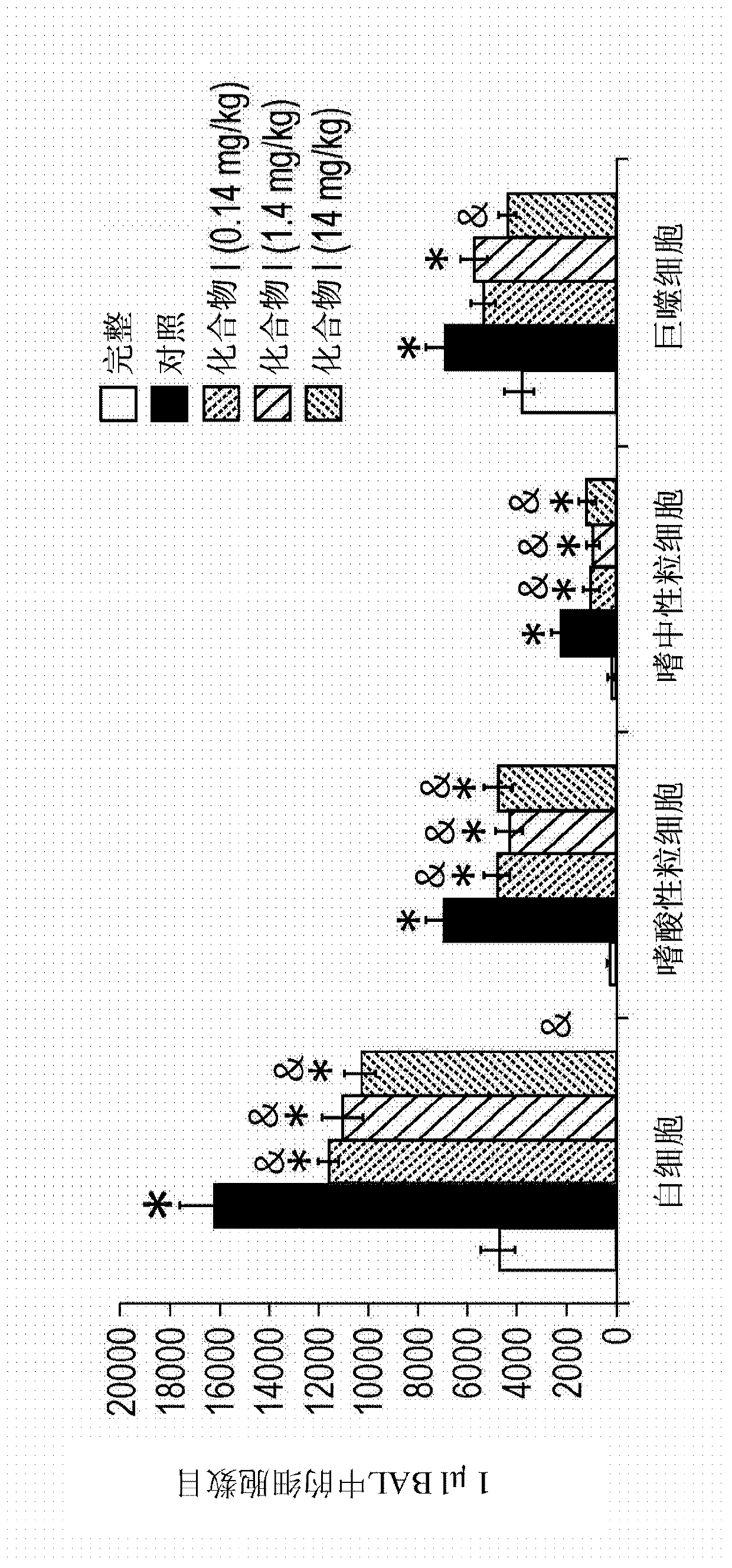
[0140] Characteristics of the biological activity of the compounds according to the invention
[0141] The biological activities of compounds I to X have been studied in different in vitro and in vivo experiments. In particular, activity studies of Compound I and Compound II in various in vitro and in vivo models have shown inhibitory effects of Compound I and Compound II on the chemotaxis of monocytes, macrophages and other immune system cells. The biological effects of Compound I and Compound II cannot be predicted or explained based on existing knowledge about the ability of Compound I and Compound II to chelate metal ions.
[0142] Studies of the biological activity of compounds III to X in vitro r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com