Artificial exosomes, preparation method and application thereof
An exosome and artificial technology, applied in the field of medicine, can solve problems such as difficulties in establishing standardized solutions, and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
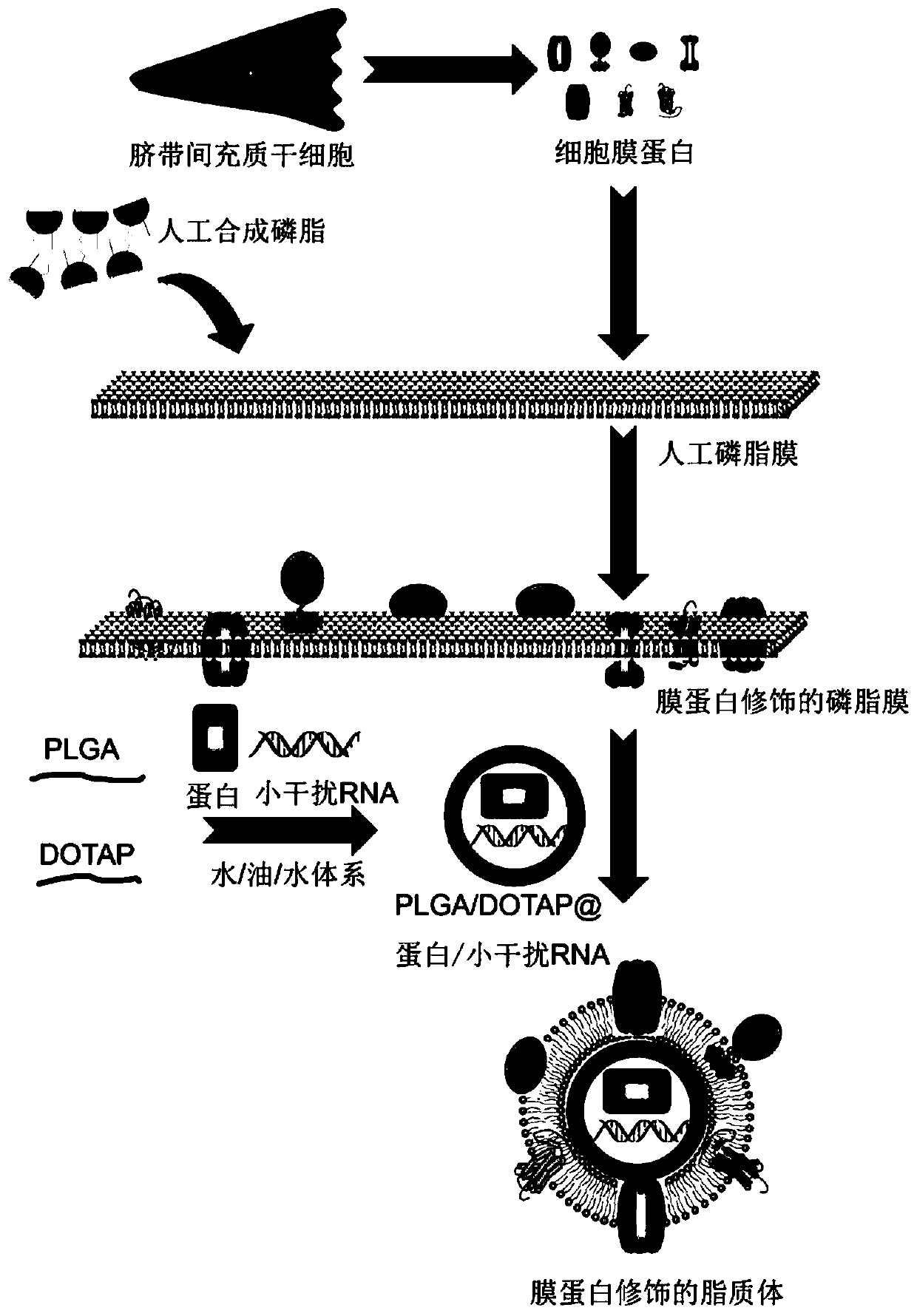
[0077] This example is used to illustrate the extraction of membrane proteins.
[0078] In this experiment, Biyuntian's cell membrane protein and cytoplasmic protein extraction kit was used.
[0079] 1) Preparation of reagents: dissolve and mix membrane protein extraction reagents A and B at room temperature, place on ice immediately after dissolving, take appropriate amount of membrane protein extraction reagents A and B for later use, add PMSF within a few minutes before use, and make The final concentration of PMSF was 1 mM. (Note: PMSF must be added within 2-3 minutes before the extraction reagent A is added to the sample, so as to avoid the rapid failure of PMSF in the aqueous solution);
[0080] 2) Culture the cells at 15cm 2 In the culture bottle, raise to 80-90% density, about 2-5×10 7 Remove the medium, wash three times with pre-cooled PBS, use a cell scraper to remove the cells, blow the cells down with a pipette, add 1mL of PBS, transfer to a 1.5mL EP tube, 4°C, ...
Embodiment 2
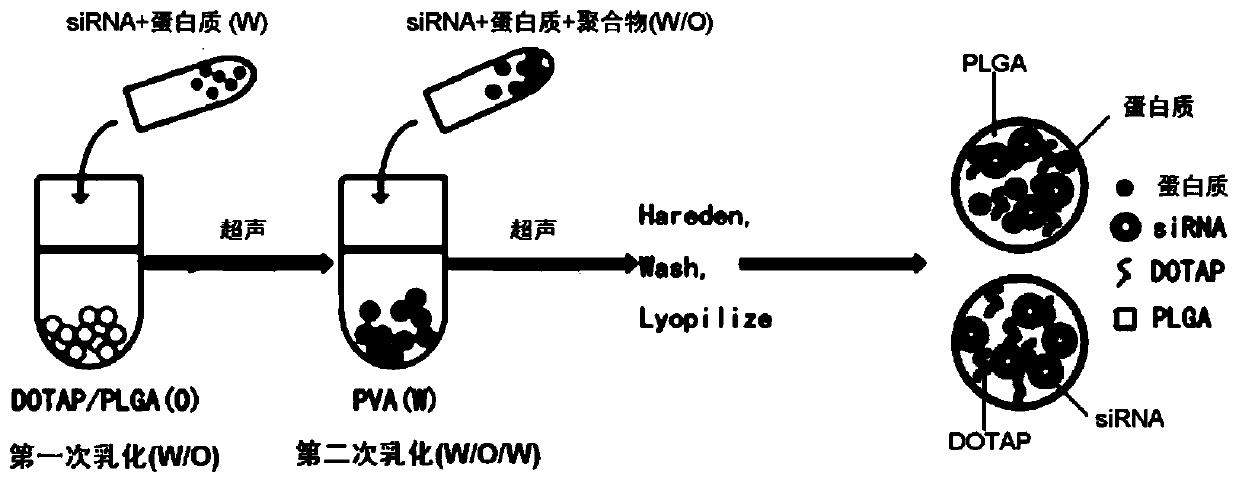
[0085] This example is used to illustrate the preparation of PLGA / DOTAP nanoparticles of the present invention, and shows the process of loading drug OCT4 protein and siTrim28 in PLGA.
[0086] Prepared by solvent evaporation and water-in-oil-in-water emulsion.
[0087] 1) The mass ratio of DOTAP to PLGA is 1:25, PLGA particles (100mg) and DOTAP powder (4mg) are dissolved in 1mL of dichloromethane, oil phase (O);
[0088] 2) Dissolve OCT4 protein (0.1mg) and siTrim28 (0.1mg) in 300uL nuclease-free water, water phase (W);
[0089] 3) Add the water phase prepared in step 2) dropwise to the oil phase prepared in step 1), while ultrasonicating for 45s, power 60%, 2s on 1s off, and operate on ice;
[0090] 4) After emulsification, add the emulsion obtained in step 3) dropwise into 7% (W / V) polyvinyl alcohol (PVA) aqueous solution (3mL), while ultrasonicating for 1min, power 60%, 2s on 1s off, in ice operation on;
[0091] 5) Add the double emulsion obtained in step 4) into 1% (W...
Embodiment 3
[0095] This example is used to illustrate the preparation of membrane protein-modified liposome-encapsulated PLGA nanoparticles of the present invention.
[0096] The thin layer evaporation (TLE) method was used.
[0097] 1) Combine 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-glycero-3-phosphocholine (DSPC), 1,2-diole Acyl-glycerol-3-phosphocholine (DOPC) and cholesterol (Cholesterol) were respectively dissolved in analytical pure chloroform: anhydrous methanol mixture (3:1 V / V) according to the molar ratio of 5:1:3:1;
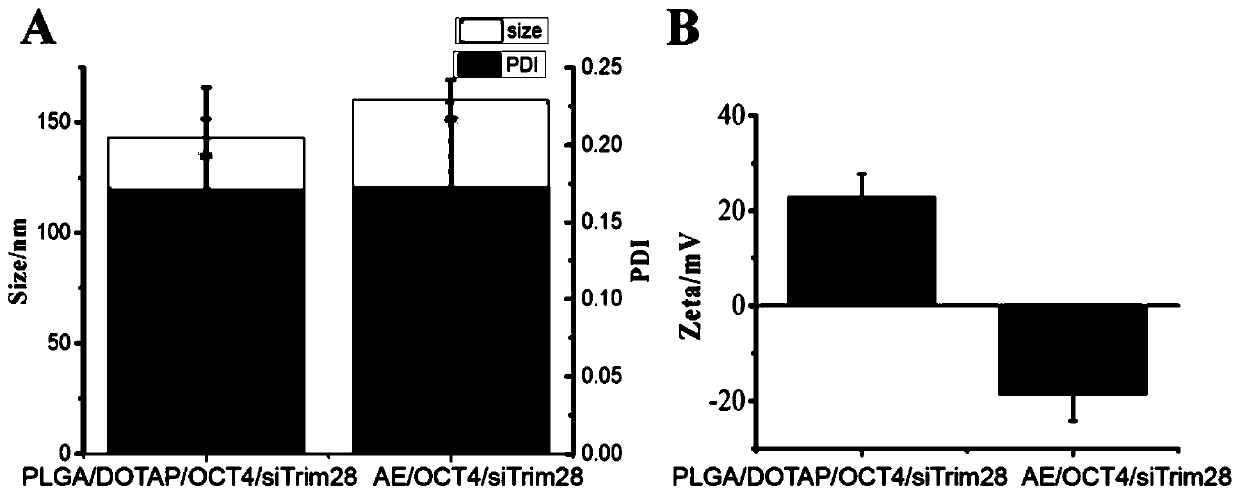
[0098] 2) The solvent of the solution obtained in step 1) was then evaporated by a rotary evaporator to form a thin film. Add different proportions of membrane proteins separated from Example 1 to the PLGA / DOTAP / OCT4 / siTrim28 nanoparticle suspension formed in Example 2 (protein: lipid mass ratio=1:100, 1:200, 1:300, 1 : 400, 1: 500), vortexed to mix, and then added to the round bottom flask to hydrate the membrane to assemble into nano...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com