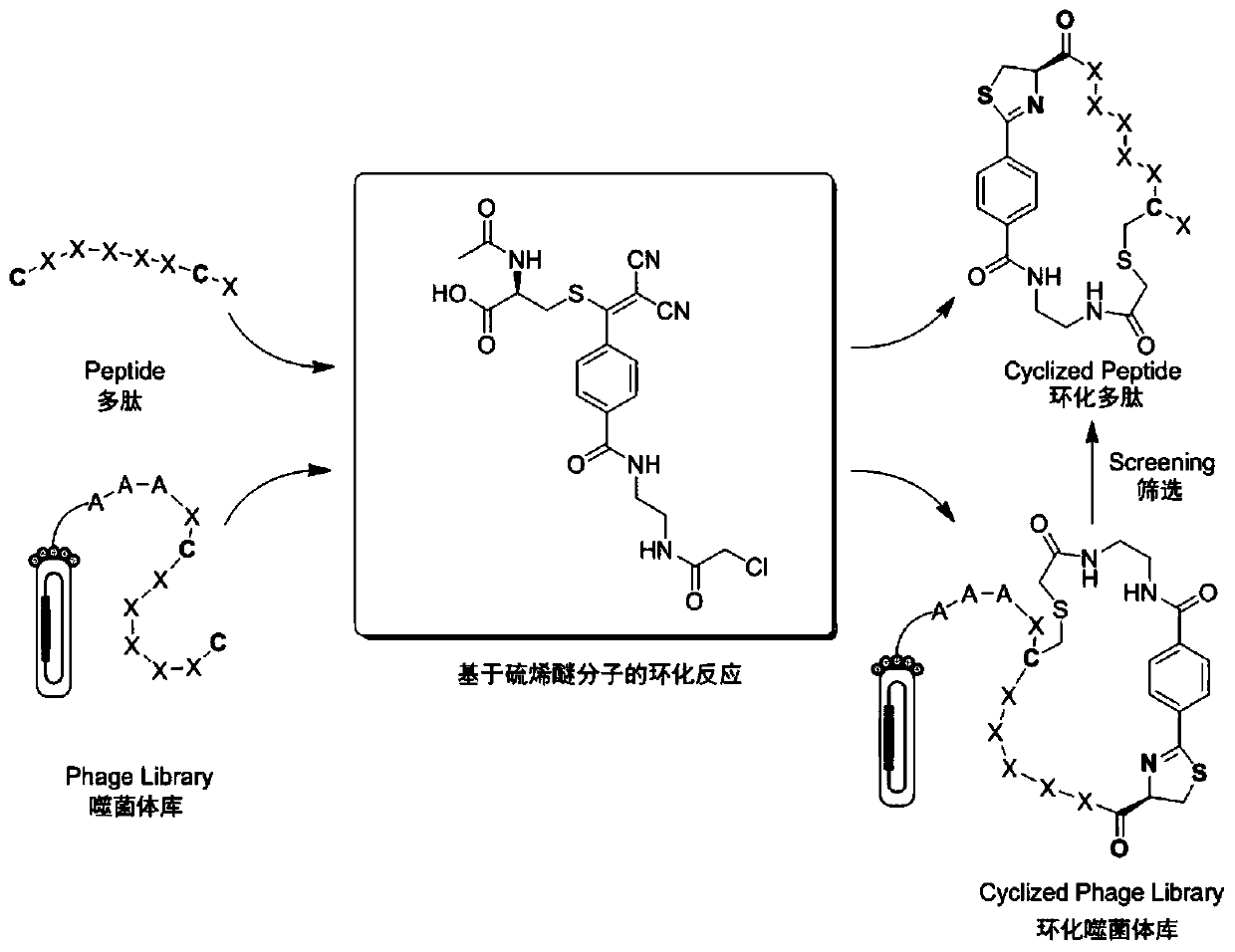
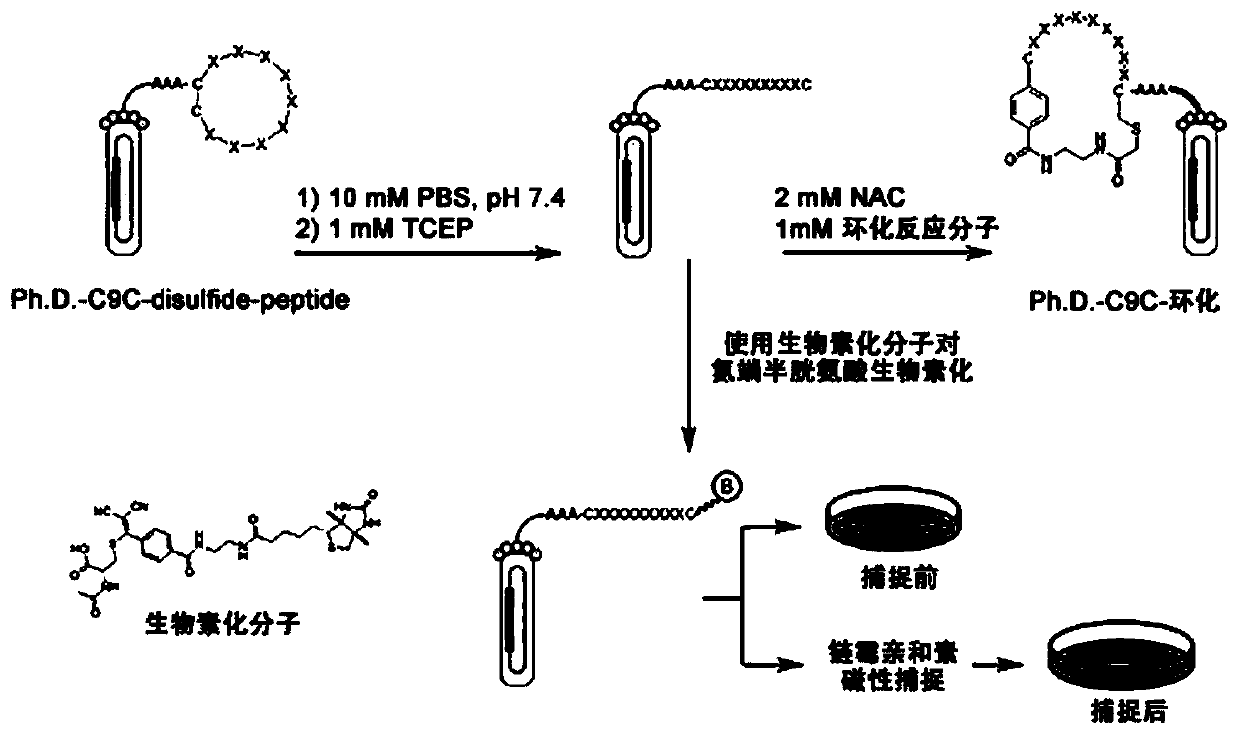
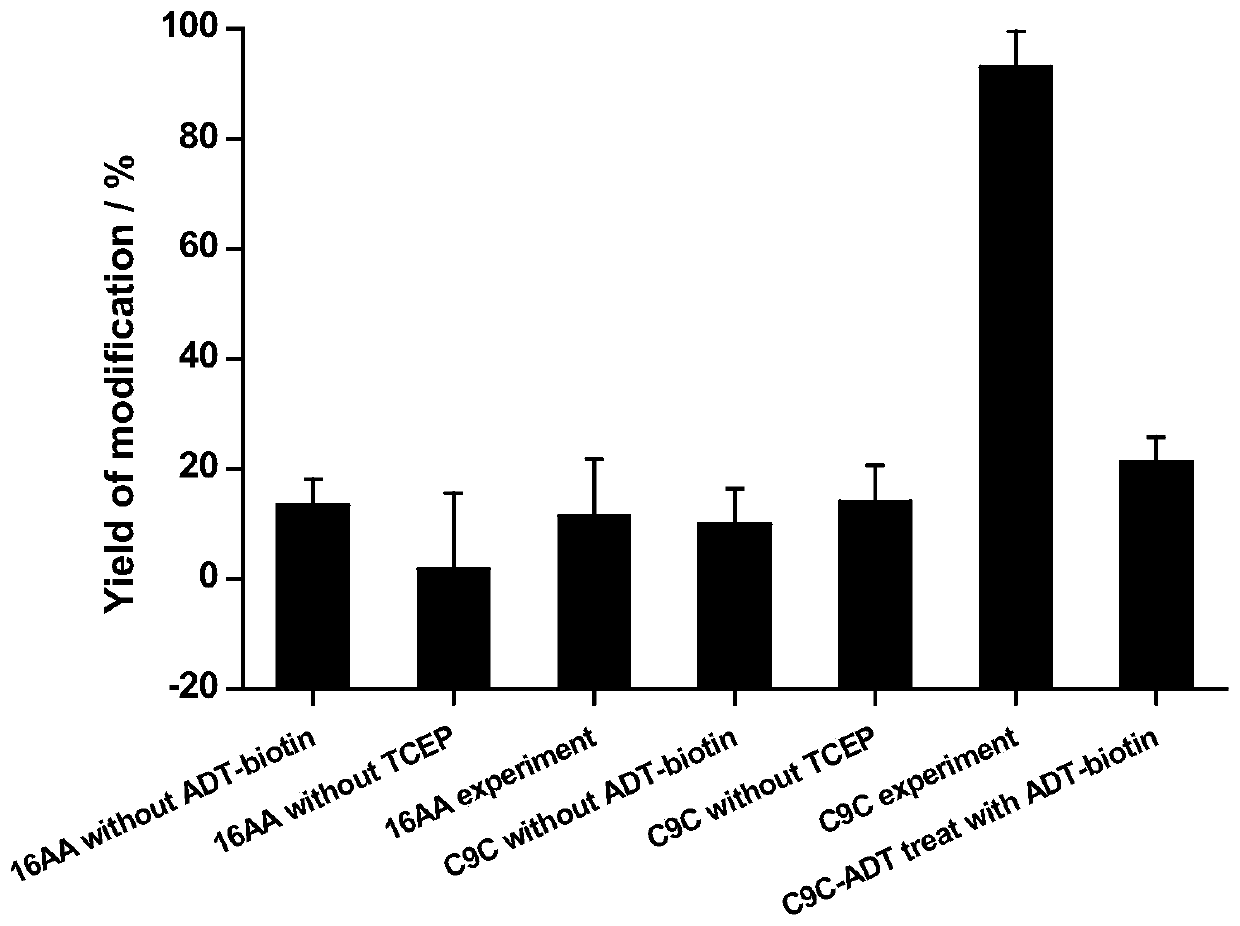
A kind of thioene ether compound and its synthesis method and application in polypeptide cyclization
A technology of sulfenyl ethers and compounds, applied in the field of biochemistry, can solve problems such as complex synthesis of cyclic peptides, and achieve good biocompatibility, green raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthesis of the thioene ether compound comprises the following steps:
[0051] Step 1, the synthesis of compound methyl 4-(2,2-dicyano-1-hydroxyvinyl)benzoate
[0052]
[0053] Specific steps are as follows:
[0054] (1) Put sodium hydride (1.8 g, 75.0 mmol) in a round bottom flask, add anhydrous tetrahydrofuran (30 mL) to disperse, and stir vigorously in an ice bath at 0 °C under nitrogen atmosphere protection. Malononitrile (2.5 g, 37.5 mmol) was dissolved in anhydrous tetrahydrofuran (30 mL), and slowly added dropwise to the above system. The reaction system was kept in an ice bath at 0° C., and stirred for 1 h in the ice bath after the dropwise addition.
[0055] (2) Methyl 4-chloroformylbenzoate (7.5 g, 37.5 mmol) was dissolved in anhydrous tetrahydrofuran (30 mL). While maintaining an ice bath at 0°C, the solution was slowly added dropwise to the system. After the dropwise addition, the reaction system was transferred to room temperature, allowed to wa...
Embodiment 2
[0097] Cyclization of Peptides
[0098]
[0099]The cyclization reaction molecule mother solution (500 μmol / L, 20 μL), peptide mother solution (500 μmol / L, 10 μL), N-acetyl-L-cysteine (5 mmol / L, 4 μL) and TCEP (5 mmol / L, 4 μL) Add to phosphate buffer (pH=7.4, 200mmol / L, 50μL), and add water (12μL). The concentration of each component in the final system is: thioene ether (100 μmol / L), polypeptide (50 μmol / L), TCEP (200 μmol / L), N-acetyl-L-cysteine (200 μmol / L) and phosphate buffer solution (pH=7.4, 100mmol / L). The system was placed at room temperature for 90 min. The reaction was monitored using high performance liquid chromatography. After the reaction, the reaction product was characterized by ultraviolet-visible absorption spectrum and mass spectrometry. Quantification according to high performance liquid chromatography, as shown in Table 1 is the characterization data of the cyclization reaction, and the conversion rate of the polypeptide is 100%.
[0100] Tabl...
Embodiment 3
[0104] Selectivity test for peptide cyclization
[0105]
[0106] The cyclization reaction molecular mother solution (500μmol / L, 20μL), polypeptide H-CGGGKGW-NH 2 Mother solution (500μmol / L, 10μL), N-acetyl-L-cysteine (5mmol / L, 4μL) and TCEP (5mmol / L, 4μL) were added to phosphate buffer (pH=7.4, 200mmol / L, 50μL ), and supplemented with water (12 μL). The concentration of each component in the final system is: thioene ether (100 μmol / L), polypeptide (50 μmol / L), TCEP (200 μmol / L), N-acetyl-L-cysteine (200 μmol / L) and phosphate buffer solution (pH=7.4, 100mmol / L). The system was placed at room temperature for 90 min. The reaction was monitored using high performance liquid chromatography. After the reaction, the reaction product was characterized by ultraviolet-visible absorption spectrum and mass spectrometry. According to the characterization results, the cysteine at the N-terminal of the polypeptide reacted with the cyclized molecule, and 100% conversion of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com